There are a lot of ways how you can achieve a strong MCAT score. A score that would get you into the medical school of your choice. One strategy is to answer practice questions, which most MCAT test-takers would agree with.
Answering practice questions gauges where you are on your MCAT preparation, whether you are on schedule or behind.
At the same time, answering practice questions gives you an idea of how questions are formulated on the MCAT. They are indeed a great way to prepare for the MCAT.
In this article, we are providing you with a few MCAT biochemistry practice questions.
What is MCAT Biochemistry?
Biological chemistry, commonly known as biochemistry, studies chemical reactions that occur inside and are connected to living things.
Biochemistry is a subfield of biology and chemistry that includes the fields of structural biology, enzymology, and metabolism. Through these three fields, biochemistry has gotten better at explaining life processes over the last few decades of the 20th century.
Understanding the chemical principles that allow biological substances to give rise to the events that occur within and between live cells is the main goal of the study of biochemistry.
Due to its importance in the field of medicine and the fact that it is essential for comprehending tissues, organs, and the structure and operation of organisms, it is included on the MCAT.
Chemical and Physical Foundations of Biological Systems and Biological and Biochemical Foundations of Living Systems are two MCAT sections that include biochemistry.
Your MCAT biochemistry knowledge and skills are required for twenty-five (25) percent of the MCAT Chem/Phys portion.
In this section, there are 59 questions total, and 15 of them are regarding MCAT biochemistry.
Additionally, 25% of the questions on the MCAT's bio/biochem section are biochemistry in nature. In this section, there are 15 MCAT biochemistry-related questions out of 59.
Summary Table of Biochemistry Distribution in the MCAT
MCAT Section | Chemistry Subject | Percentage | Number of Questions (out of 59) |
|---|---|---|---|
Chemical and Physical Foundations of Biological Systems | First-Semester Biochemistry | 25% | 15 |
Biological and Biochemical Foundations of Living Systems | First-Semester Biochemistry | 25% | 15 |
Total Number of MCAT Organic Chemistry Questions: 30 | |||
Biochemistry Topics to Study for the MCAT
You need to be familiar with the wide range of subjects covered in MCAT biochemistry to prepare for the MCAT adequately.
Before taking the MCAT, ensure that you have mastered these concepts to get the score that you aim for.
The following are the different MCAT biochemistry topics covered in the MCAT:
MCAT Biochemistry Practice Questions
Nobody would contest the notion that answering test questions before the actual MCAT is very beneficial for test takers.
By taking practice exams, you can get accustomed to the questions' structure and the variety of possible responses. You will start to notice trends in questions (and answers) and subsequently master how to answer them. They also evaluate your level of readiness and offer assistance as needed.
Here are a few sample practice questions for the MCAT Biochemistry section.
1. Which of the following claims about nonpolar R groups in an aqueous solution is most likely accurate?
A. They hide inside proteins and are hydrophilic.
B. They are hidden within proteins and are hydrophobic.
C. They are present on protein surfaces and are hydrophilic.
D. They are present on protein surfaces and are hydrophobic.
2. The "triple helix" structure of collagen is made up of three carbon-based helices that are tightly coiled around one another. Which of these amino acids is most likely to be present in collagen in the largest amounts?
A. Proline
B. Glycine
C. Cysteine
D. Threonine
3. Which of the following claims about peptide bonding is FALSE?
A. They have a partial double-bond personality.
B. Hydration reactions are involved in their production.
C. They serve as the main ties that connect amino acids.
D. They are created when an amino group and a carboxyl group react.
4. Substrate C is an allosteric inhibitor of enzyme 1 in the equation below. Which of the following mechanisms is a necessary consequence of substrate C?
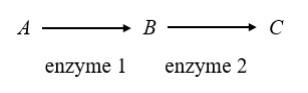
A. Negative feedback
B. Irreversible inhibition
C. Competitive inhibition
D. Feedback enhancement
5. Which of the following DOES NOT constitute an effect of enzymes that lowers the activation energy of biological reactions?
A. Creating momentary covalent connections
B. Changing the immediate environment of charge
C. Taking on the roles of electron donors or receptors
D. Breaking the bonds in the enzyme to release energy
6. Four subunits make up a specific cooperative enzyme, and two of them are bonded to the substrate. Which of the aforementioned statements is TRUE?
A. The enzyme's affinity for the substrate has recently reduced.
B. The enzyme's affinity for the substrate has just been stronger.
C. For this enzyme class, the enzyme's substrate affinities are average.
D. The enzyme has a higher affinity for the substrate when more than one substrate is bound.
7. When an enzyme catalyst is present versus when it is absent, how does the reaction's optimum temperature change?
A. The optimum temperature is lower with a catalyst than without.
B. The reaction, not the enzyme, determines the optimal temperature.
C. Without understanding the type of enzyme, no conclusion can be drawn.
D. With a catalyst present, the optimum temperature is typically higher than without one.
8. Hormones are present in the body in very little amounts, but they frequently have a significant impact. What kind of receptor is most likely to be acted upon by hormones?
I. Ligand-gated ion channels
II. Enzyme-linked receptors
III. G protein-coupled receptors
A. I only
B. III only
C. I and III only
D. I, II, and III
9. Which of the aforementioned traits is NOT ascribed to antibodies?
A. Antibodies bind to numerous unique antigens.
B. Antibodies contain both two heavy and two light chains.
C. Antibodies identify antigens for other immune cells to target.
D. Agglutination can be brought on by antibodies interacting with antigens.
10. What distinguishes the gel used in isoelectric focusing from the gel used in conventional electrophoresis?
A. In isoelectric focusing, a pH-gradient gel is used to promote a changeable charge.
B. To allow for full migration, isoelectric focusing uses a gel with substantially wider pore diameters.
C. The protein mixture is treated before loading; the gel is unaffected during isoelectric focusing.
D. To promote a homogeneous negative charge, isoelectric focusing uses a gel to which SDS has been added.
11. The structure of glucose's C-4 epimer, galactose, is seen here. Galactose is found in which of the following structures?
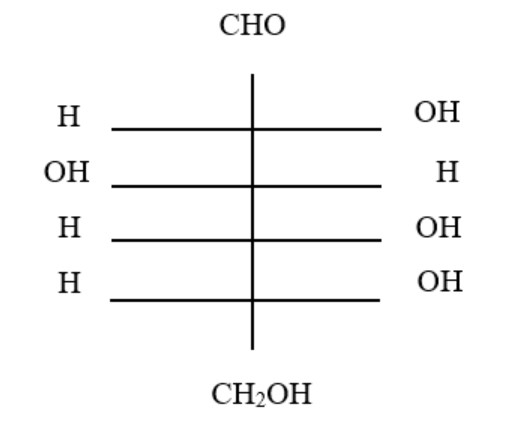
A.
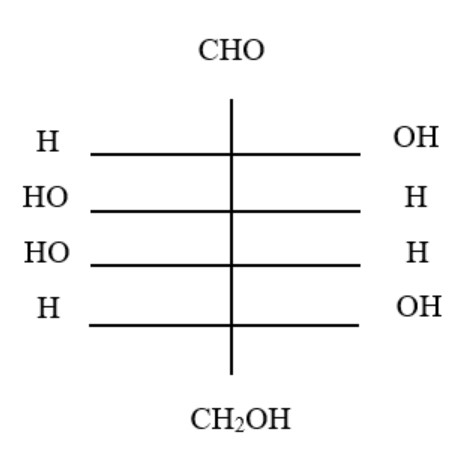
B.
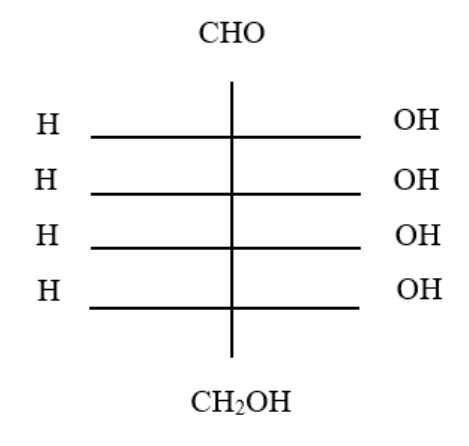
C.
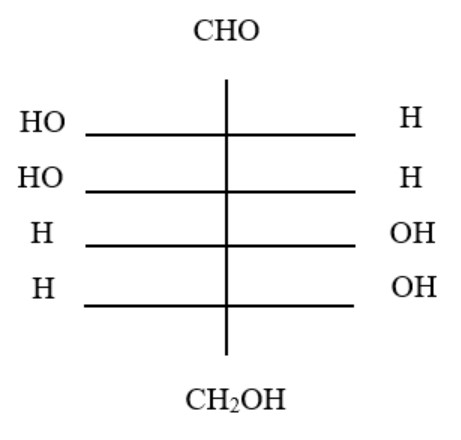
D.
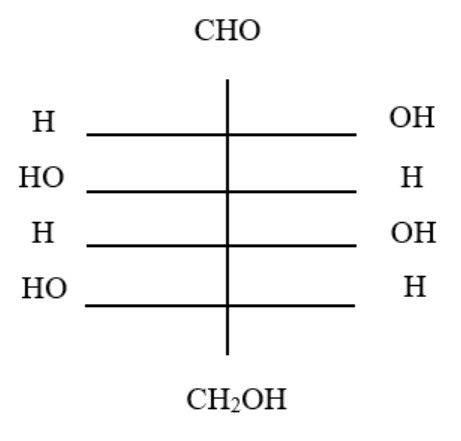
12. possible for ketose sugars to function as reducing sugars. What mechanism explains this?
A. Anomerization occurs with ketose sugars.
B. Tautomerization occurs with ketose sugars.
C. The ketone group undergoes direct oxidation.
D. The ketone group undergoes a direct reduction.
13. Which statement concerning fat-soluble vitamins is TRUE?
I. The control of calcium depends on vitamin E.
II. Since vitamin D is a biological antioxidant, it prevents cancer.
III. Calcium-binding sites must be introduced post-translationally, which requires vitamin K.
IV. Retinal, which is crucial for vision, is created when vitamin A is digested.
A. III only
B. I and II only
C. III and IV only
D. II, III, and IV only
14. Which of the following aspects of gene sequencing raises ethical questions?
A. Gene sequencing affects families; therefore, privacy issues can be brought up.
B. Gene sequencing is incredibly imprecise, which heightens worry over the results.
C. False-negative results from gene sequencing may give one a false sense of security.
D. Because gene sequencing is an invasive procedure, potential health concerns need to be fully disclosed.
15. Why might uracil be kept out of DNA but not RNA?
A. Uracil is produced by the breakdown of cytosine.
B. Uracil is employed as an activator of DNA synthesis.
C. Replication is hindered by uracil's excessive adenine binding.
D. The synthesis of uracil is substantially more challenging than that of thymine.
MCAT Biochemistry Sample Passage 1
Neurodegenerative diseases include Alzheimer's disease (AD). The misfolding and conformational alterations of the amyloid beta protein (A), which is localized in neuronal synapses, are one of the pathogenic processes in the development of AD.
A has physiological significance and is primarily created by the amyloidogenic metabolism of the amyloid precursor protein, which is accomplished by the sequential action of - and -secretases and results in the liberation of a peptide between 39 and 42 amino-acid residues.
However, in a sick state, amyloid beta production outpaces its removal from the body, causing target proteins to become toxic after changing from a -helix to a -sheet structure. Such a bizarre structural change encourages protein aggregation by exposing hydrophobic amino acid residues. Senile plaques, also known as A40 and A420, are extracellular deposits of A that are primarily 40 or 42 amino acids long.
The A40 version is the more prevalent of the two, however, A42 is more fibrillogenic and thus linked to AD due to the additional two hydrophobic C-terminus amino acids (isoleucine and alanine). The researchers discovered stable parallel and antiparallel -sheets within the amyloid fibrils in a study on the disease-associated mutation, (D23N) A40.
Recently, several small chemical probes that can monitor and/or inhibit protein aggregation have been created. It has been discovered that artificial peptide derivatives known as "-sheet breakers" can limit the production of amyloid by attaching to monomeric precursors or by preventing fibril elongation by blocking fibril ends.
Prolines or amino acids with N-methyl modifications can be used to replace specific residues in synthetic peptides that correspond to the amyloid core areas in order to prevent the formation of hydrogen bonds, which are necessary for the cross-structure. Due to their tiny size and lack of peptide connections, small molecules have a number of benefits over peptide-based compounds.
16. What leads to the accumulation of amyloid beta peptide in the extracellular space during illness?
A. Amyloid peptide conformational alterations
B. The body's amyloid peptide slowly leaves it
C. Excessive amyloid beta cleaving enzyme activation
D. Amyloid peptide cleavage at the amyloid precursor protein's C-terminus
17. Why do peptide-based beta sheet breakers not have the same advantages as small molecule inhibitors of protein aggregation?
A. They have no trouble crossing the blood-brain barrier.
B. They can withstand hydrolysis in the body, in contrast to peptide-based medications.
C. It is simple to conduct tests on the structure-activity relationship to choose the optimum aggregation inhibitor.
D. Both A and B
18. Why is any protein's beta-sheet structure more prone to aggregation?
A. The hydrophobic amino acids are abundant in it.
B. The hydrophobic amino acids come into contact with water.
C. In general, alpha helix conformations are less stable than beta-sheet conformations.
D. It interacts with the surrounding aquatic environment through an exposed hydrophilic area.
MCAT Biochemistry Sample Passage 2
The blood pH of the human body must be kept between 7.35 and 7.45 for a healthy operation. Large departures from this range are quite risky. Acidosis is a term used to describe any situation in which the blood pH falls below 7.35. Alkalosis occurs when the pH exceeds 7.45.
The proper operation of the body's chemical buffer system in the blood together with the respiratory and urinary systems is crucial for preventing acidosis or alkalosis. The blood plasma's chemical buffer system includes buffers for carbonic acid, phosphate, bicarbonate, and plasma proteins.
Bicarbonate in the blood is controlled by sodium in the bicarbonate-carbonic acid buffer system. Carbonic acid (H2CO3), a weak acid, and sodium chloride (NaCl) are produced when sodium bicarbonate (NaHCO3) reacts with a strong acid, such as HCl. Bicarbonate and water are created when carbonic acid reacts with a potent base, like NaOH.
At a biological pH, the blood contains carbonic acid and bicarbonate ions in a 20:1 ratio. This capture mechanism is particularly effective at buffering changes that would make the blood more acidic since it contains 20 times as much bicarbonate as carbonic acid.
Since the majority of the body's metabolic waste products, such lactic acid and ketones, are acids, this is helpful. The blood's level of carbonic acid is regulated by CO2 exhalation through the lungs. The renal system regulates the amount of bicarbonate in the blood by conserving and reintroducing bicarbonate ions from the renal filtrate into circulation.
By controlling the quantities of carbonic acid in the blood, the respiratory system helps the body's acid-base balance. The levels of CO2 and carbonic acid in the blood are in equilibrium. Carbonic acid is easily formed when CO2 in the blood combines with water.
Blood pH is lowered when the amount of CO2 in the blood increases (as it occurs when you hold your breath). The extra CO2 combines with the water to produce more carbonic acid. You can exhale more CO2 by speeding up and/or deepening your breathing (which you may feel "urged" to do after holding your breath). The blood's amount of carbonic acid is decreased by the body's release of CO2, which raises the pH toward normal ranges. This process also operates in the reverse direction, as you would have guessed.
Hyperventilation, or excessively deep and rapid breathing, causes the blood to become too alkaline by removing CO2 from the body and lowering the level of carbonic acid. Rebreathing air that has been exhaled into a paper bag can treat this transient alkalosis. Re-inhaling exhaled air will quickly return blood pH to normal.
19. Which of the following pairs is a suitable candidate for a buffer system?
A. HCl / NH4OH
B. NaOH / NaCl
C. NH4OH / NH4Cl
D. CH3COOH / NaOH
20. The pH of the blood affects hemoglobin's (Hb) capacity to transport oxygen as oxyhemoglobin (HbO2) throughout the body. How would acidity affect a patient's capacity to transfer oxygen?
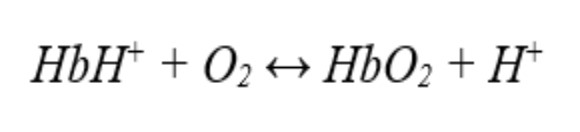
A. Remains the same
B. Increases first, then drops
C. Improves oxygen transport capacity
D. Reduces the capacity to transport oxygen
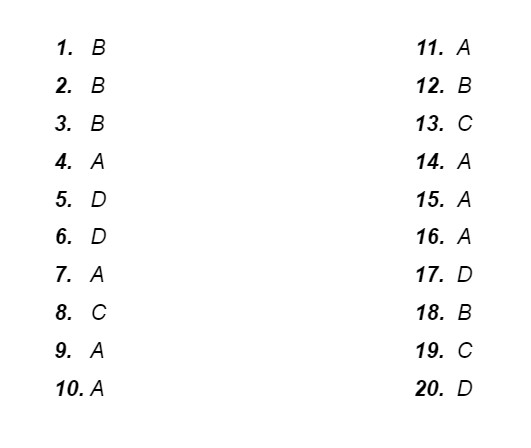
Answer Key:


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 




















