I. What are the Fundamentals and Key Terms of Glycolysis?
To make a real world analogy, if the various MCAT content and topics were like children, this would definitely be one of the AAMC and MCAT’s favorite children, probably giving it a bit more attention and love than the other subjects, haha!
When looking at it from a biochemical perspective, it’s no wonder that this would be one of the favorite kids as glycolysis is an essential, necessary process that all cells must possess in order to power the supply of the cell and all of its processes.
We have to apologize as this is the section which requires a decent amount of memorization
II. Overview of Glycolysis
Let’s first start off with an overview of glycolysis before getting into the actual enzymes and processes discussed in another section!
A. Net Molecular and Energetic Results of Glycolysis
Glycolysis is the process where 1 glucose molecule is oxidized (loses electrons) to produce 2 pyruvate molecules, all of this occurring in the cytoplasm. Viewing glycolysis as an electron transfer reaction is helpful, as it’ll come up when discussing the importance of the resulting products.
Think of glycolysis like an equation! Glucose has 6 carbons & pyruvate has 3 carbons: thus, it works out that 1 glucose yields 2 pyruvates as the number of carbons will be equal.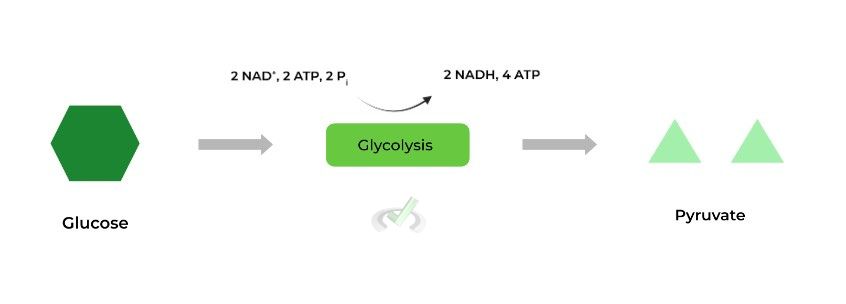
In addition to pyruvate, the other major important molecules produced from glycolysis are NADH and ATP – let’s learn more about them and their importance.
I. NADH
An abbreviation for nicotinamide adenine dinucleotide, NADH is produced in glycolysis from its NAD+ precursor where it gains the electrons lost from glucose oxidation.
The addition of electrons allows NADH to function as a high energy, electron carrier! This stored energy can then be used to power the generation of ATP in oxidative phosphorylation.
Some of the glycolytic enzymes are classified as oxidoreductases due to their role in catalyzing the transfer of electrons, as you’ll see in other articles!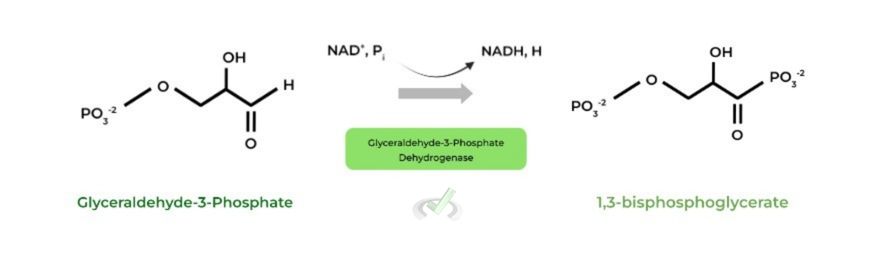
II. ATP
Standing for adenosine triphosphate, this molecule functions as one of the cell’s main sources of energy to power various cellular processes due to it being a high energy molecule.
An important term to describe glycolytic ATP production is substrate level phosphorylation: this describes the generation of ATP by the addition of a substrate bound phosphate group to ADP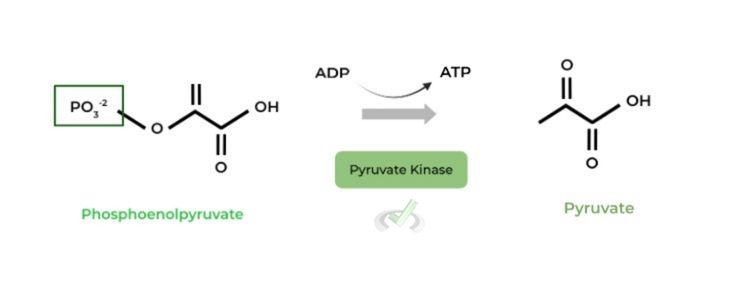
Notice how in the diagram, the source of the phosphate comes from the substrate as opposed to a free phosphate molecule!
B. Aerobic v.s. Anaerobic Glycolysis – Substrates and Products
These terms describe the glycolysis and the resulting course of action under the presence or absence of oxygen, respectively.
Aside from the type of O2 environment, 2 main ways aerobic & anaerobic glycolysis differ are the following pathways pyruvate can enter and the net ATP production. Let’s take a closer look at each of them!I. Oxidative Phosphorylation v.s Lactate Fermentation
In the presence of oxygen (aerobic glycolysis), the pyruvates can enter the Krebs Cycle and eventually undergo oxidative phosphorylation via the electron transport chain (ETC), producing much more ATP!
Oxygen is needed for oxidative phosphorylation as it’s the final electron acceptor of the ETC!
In the lack of oxygen (anaerobic glycolysis), the pyruvates enter a separate pathway called lactate fermentation as there is no O2 to act as the final electron acceptor of the ETC!
While producing lactate, the process also regenerates the NAD+ needed for glycolysis. This can now reenter glycolysis to be used to complete the process with the main goal of ATP production!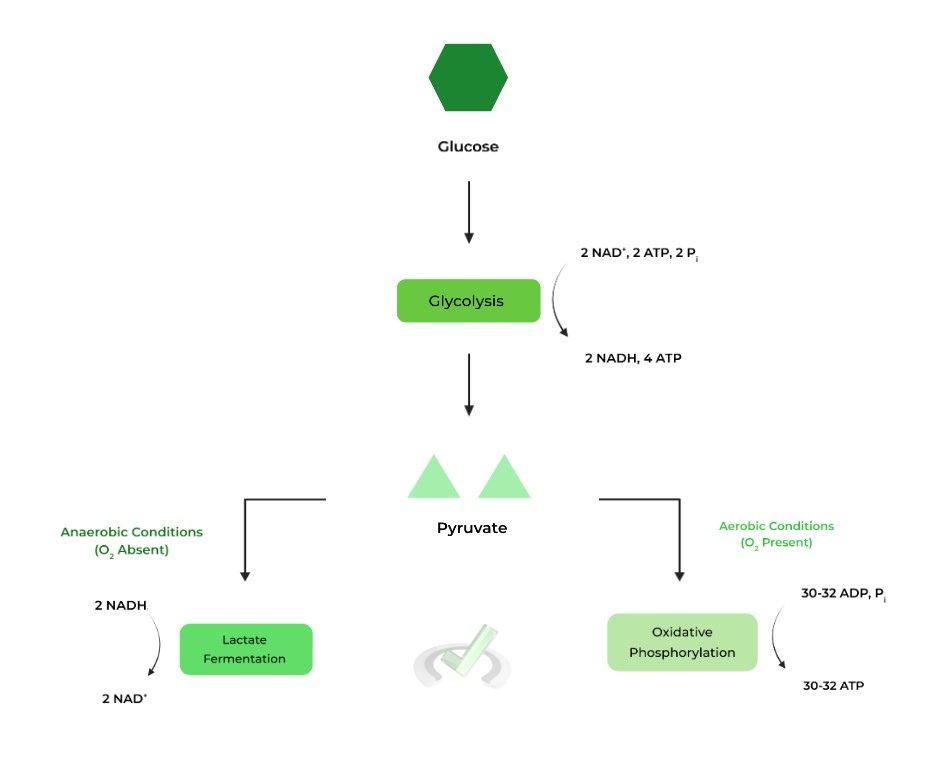
II. Net Total ATP Production
Funny enough, there is a little math that’s involved in glucose metabolism! The net total ATP production accounts for the “investment” molecules when calculating the total yield!
These are the ATP molecules that must be put in or “invested” in glycolysis in order to generate ATP. As shown above, 2 ATP molecules are invested in order to generate 4 ATP molecules.
In aerobic glycolysis, the ATP from oxidative phosphorylation must also be accounted for, resulting in a 30-32 net total ATP production. In anaerobic conditions, the net total ATP is only 2 because of no additional ATP from oxidative phosphorylation.
In order to calculate the net total of any particular product, use the equation below! We’ll also show an example of one calculation with anaerobic glycolysis!
Total Net ATP = Total ATP Produced - Total “Invested” ATP
III. Bridge/Overlap
As mentioned above, it might be helpful and beneficial to think of glycolysis as one big oxidation-reduction reaction! It also might be helpful to review some of these terms and their basic definitions.
A. Oxidation-Reduction Reactions
In its most simplest form, oxidation refers to the loss of electrons in a molecule while reduction refers to the gain of electrons. To remember this, use the well-known mnemonic: OIL-RIG!
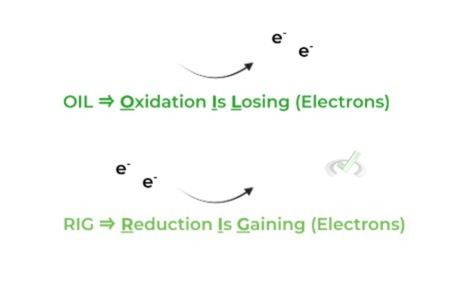
Furthermore, review the terms oxidizing agent (oxidizer) and reducing agent (reducer), but be careful! These can get tricky because of the naming but simply think about it like this:
The type of electron transfer that occurs for a molecule will be the opposite to its designation. For example, the oxidizing agent (oxidizer) will be reduced in the reaction – notice how the electron transfer is opposite to its name!
As such, the reducing agent (reducer) will be oxidized in the reaction. Let’s look at a sample step from glycolysis to apply these terms!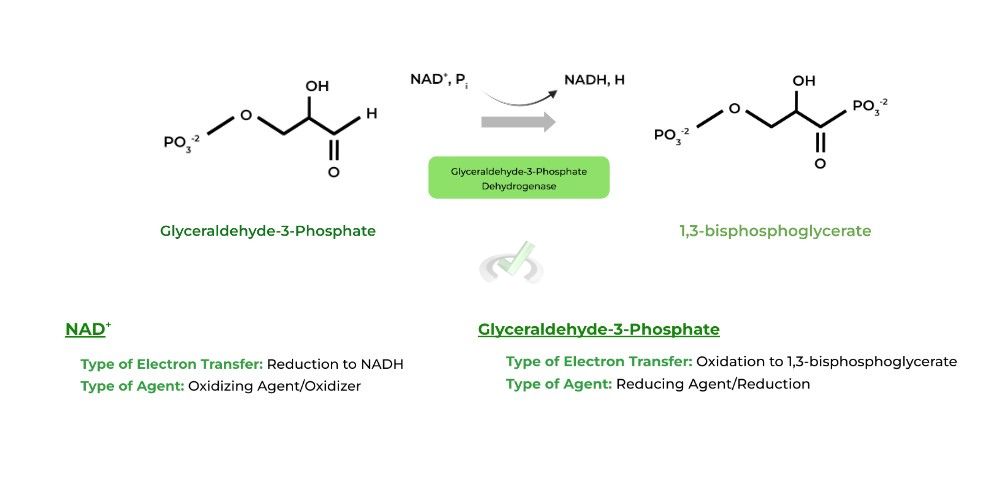
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Net Molecular and Energetic Results of Glycolysis
Glycolysis is the oxidation of 1 glucose (6 carbons) molecule into 2 pyruvate (3 carbons) molecules, a process which occurs entirely in the cytoplasm. It may also help to view glycolysis as a big oxidation-reduction reaction!
I. NADH
Stands for nicotinamide adenine dinucleotide. NAD+, the precursor molecule, gains electrons from the oxidation of glucose to produce NADH.
NADH functions as a high energy, electron carrier which can use its stored energy to power ATP production in oxidative phosphorylation!II. ATP
Stands for adenosine triphosphate and functions as one of the cell’s main energy sources. In glycolysis, ATP is produced via substrate level phosphorylation where a substrate bound phosphate group is transferred to ADP to generate ATP!
B. Aerobic v.s. Anaerobic Glycolysis – Substrates and Products
Refers to whether glycolysis takes place in the presence or absence of O2, respectively. They also differ in the following pathways after glycolysis and the net total ATP production.
I. Oxidative Phosphorylation v.s. Lactate Fermentation
Aerobic glycolysis can undergo the Krebs cycle and oxidative phosphorylation to generate more ATP, as O2 is required as the final electron acceptor in the ETC!
Anaerobic glycolysis can only undergo lactate fermentation which regenerates the NAD+ to be used for another round of glycolysis.
Because no O2 is present to be the final electron acceptor, anaerobic glycolysis CANNOT undergo oxidative phosphorylation.
II. Net Total ATP Production
When calculating net total ATP, simply take the number of total ATP generated and subtract it with the number of “invested” ATPs.
The net total ATP production differs between aerobic and anaerobic glycolysis because aerobic conditions can generate much more ATP via oxidative phosphorylation while anaerobic conditions cannot.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
If 3 glucose molecules undergo glycolysis, how many pyruvates are generated and how many carbons total atoms are present from the pyruvates?
A. 4, 18
B. 6, 12
C. 4, 6
D. 6, 18
Ans. D
Because 1 round of glycolysis generates 2 pyruvates, 3 rounds of glycolysis should generate 6 pyruvates. Likewise, the total amount of carbons from the pyruvates should equal the amount of carbons in the glucose molecules!
here were 3 glucose molecules, so there should be 18 carbons accounted for.
Sample Practice Question 2:
Which of the following amino acids is most likely to be situated on the surface of the glycolytic enzymes?
A. Ser
B. Val
C. Phe
D. Ala
Ans. A
Glycolysis takes place in the cytoplasm, a highly polar environment! As such, hydrophilic amino acids, such as those that are polar and charged (positive & negative) are the ones most likely to be localized on the enzyme’s surface!


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these