It comes without a doubt that we’re exposed to gasses every day! From the O2 that fills our lungs when we take a calming breath to the unfortunate pollution affecting the environment, gasses are heavily involved in our daily lives.
The gas phase of matter gets its own special chapter within our General Chemistry module as it has various features that make it unique and quite different from the solid and liquid phase of matter.
This chapter overview will cover the basics of gasses as a state of matter, as well as some important rules to describe their behavior. Let’s go!
The Gas Phase on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try and expect around 3-4 questions covering gasses that can be tested come Test Day. Try to stick to the main ideas and concepts as always to make your study and prep as efficient as possible!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: The Gas Phase
In general, concepts and theories on the gas phase are relatively straightforward and easy to understand! Likewise, the equations and calculations involved in gasses are relatively simple as well!
It’s more so understanding when to use these equations and apply these concepts towards passage and fundamental discrete questions that are a little more difficult. As you practice and get more comfortable with these practice problems, you’ll see key phrases and words that will help you identify when to apply and use these equations!
1. Important Terms and Variables for Gases
Probably the most common way to describe the gaseous state of matter is as a substance that contains no definite shape and no definite volume! Due to their nature, they can take on the shape and volume of any container they’re placed into!
This is on account of the fact that the gas particles are constantly moving and have large spaces between each other. Combined with the fact that they have a lower intermolecular attraction to one another, they can take on any shape or volume.
While there are many important terms to associate with gasses, pressure is probably the most important because it can tell us a lot about the nature of gas: the average kinetic energy, the number of moles in a gas, etc. The pressure of a gas is defined as the ratio between the force applied and the area of the container.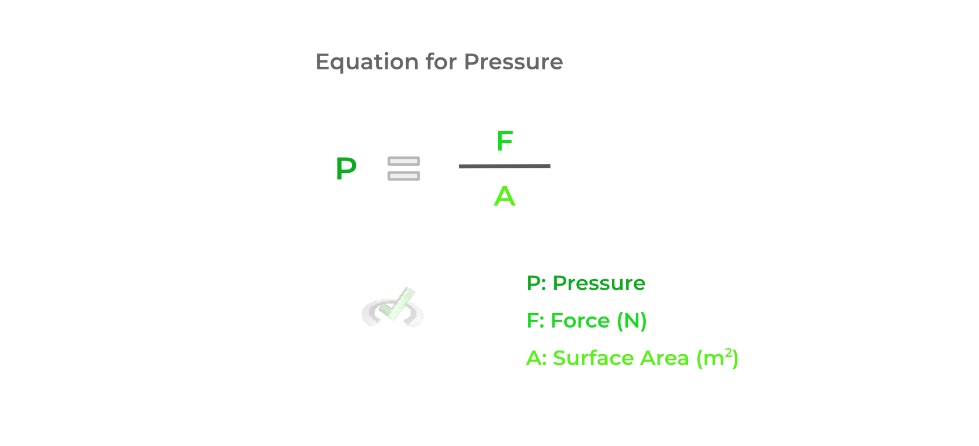
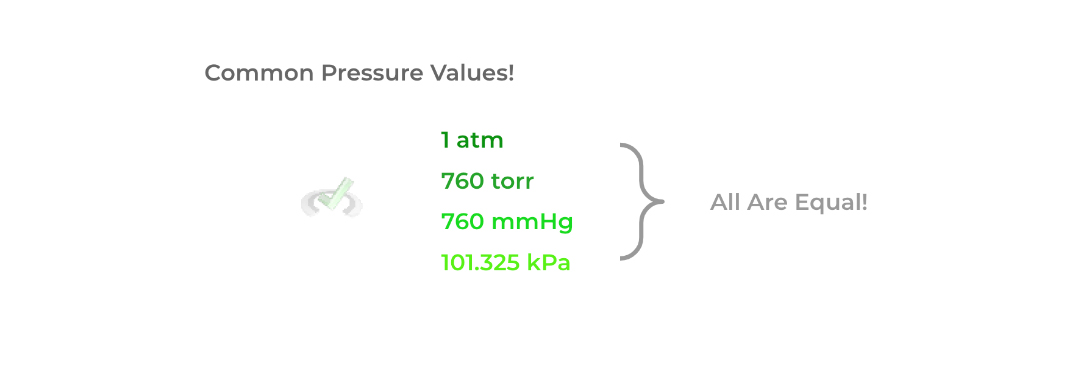
In addition, just like how there are different units to measure distance (i.e. meters, inches, yards, etc.), there are also different units we can use to measure pressure! Shown below are some of the common ways to express pressure with their conversions.
(Coming Soon!) Full Study Notes : Important Terms and Variables for Gases on the MCAT
For more in-depth content review on the different terms and variables used to describe and study gasses, check out these detailed lesson notes created by top MCAT scorers.
2. Ideal v.s. Real Gasses
Just like we create our perfect basketball player in MyCareer when playing 2K, chemists have certain assumptions about the behavior of gasses when they want to study them and make various calculations, which makes them ideal!
There are 2 main assumptions that we make when we consider gasses “ideal”: 1) the gas particles have no intermolecular forces acting between each other and 2) the gas particles themselves occupy no space. Let’s explain these assumptions!
As we’ll explain later in kinetic molecular theory, we study gas particles as constantly moving particles. When these particles collide with one another, we also assume that the collisions are elastic — if intermolecular forces are significant between the particles, this would reduce the “elasticity” of the collisions.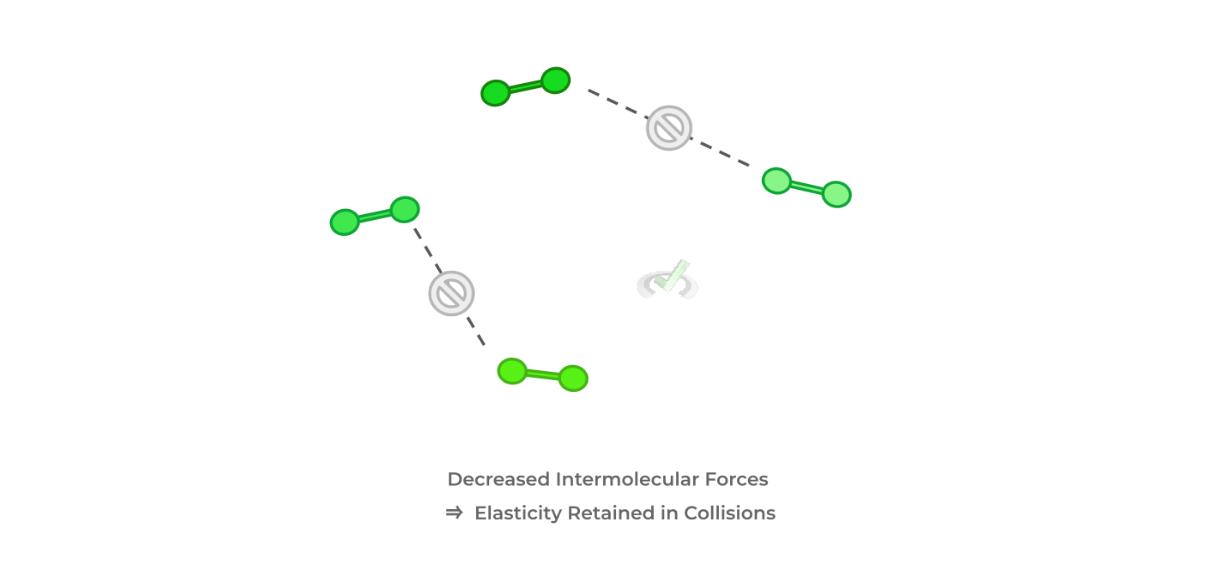
The second point is a little bit confusing as it goes against common sense. One way to think of the gas particles is that the size of the particles themselves is negligible, which is another way of saying they occupy no space!
Note that the gas will still take up the entire volume of the container they’re placed in; however, we’re just assuming that the gas articles themselves aren’t occupying space. Specifically, the smarty pants chemists refer to them as “massless point particles” to further emphasize the negligible volumes of gas particles in ideal gasses.
Unfortunately, there can never be a perfect MyCareer player in real life as there are always deviations and limitations. Similarly, real gasses under certain conditions will tend to deviate from the behaviors assumed for ideal gasses.
There are 2 conditions where gasses begin to deviate from ideal gasses: under 1) high pressure and 2) low temperature. In both cases, the particles will have significantly stronger intermolecular forces, breaking our first condition for ideal gasses.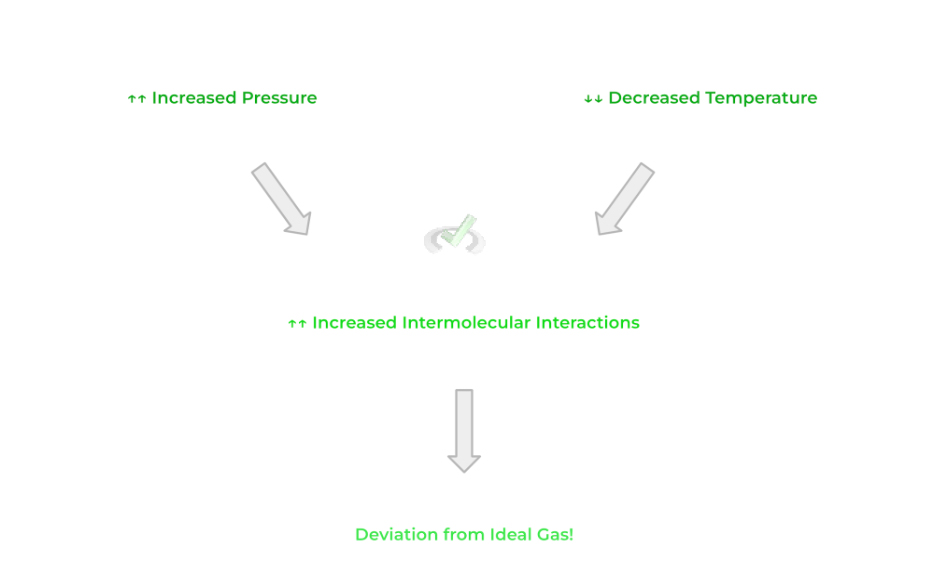
(Coming Soon!) Full Study Notes : Ideal v.s. Real Gasses on the MCAT
For more in-depth content review on the difference between ideal and real gasses, check out these detailed lesson notes created by top MCAT scorers.
3. High Yield Gas Laws
Over the years of scientific study, many great chemists have found many important relationships in gasses and have developed equations and laws that are definitely high yield for the MCAT, which we’ll introduce in this section!
It’s best to start by first introducing the ideal gas law, as we can use this as a basis to derive the other laws covered. As shown below, the law gives a relationship between the pressure (P), volume (V), moles (n), and temperature (T) of a gas!
The “R” variable in the equation is called the ideal gas constant equal to 0.0821 L*atm/mol*K. The value of 8.314 J/mol*K is also used, but the former is much more common as it contains the units of all the variables included in the ideal gas law.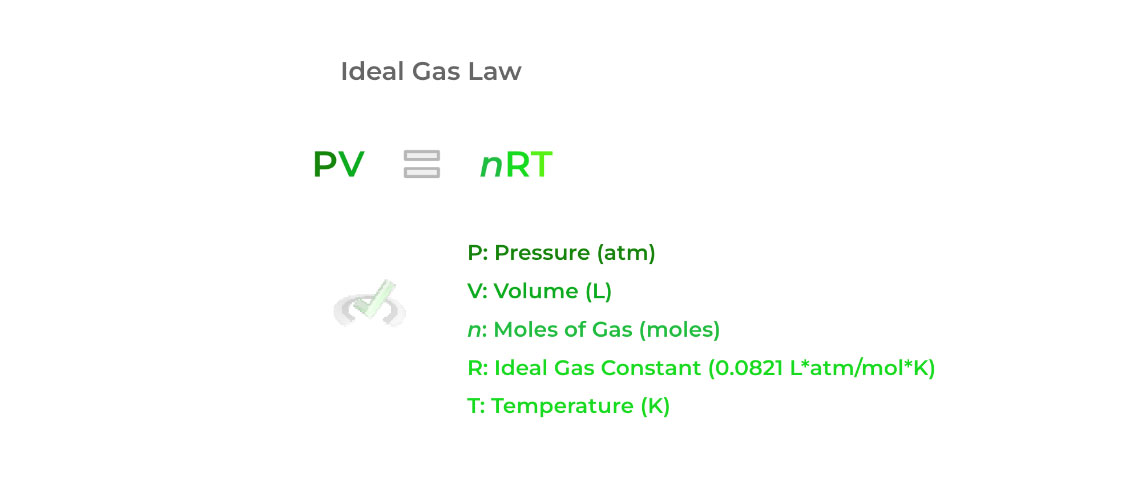
The great benefit of this equation is that if we’re missing one value of one of the variables from the equation, we can solve for it! By getting the term to one side with some basic algebra, we can go ahead and solve for the missing variable!
From this equation, we can derive some of the other high yield gas laws — the important aspect of these laws is understanding the relationship between the 2 variables.
Additionally, because the laws that we’ll discuss below have only 2 variables changing from the ideal gas law, the other variables of the ideal gas law will remain constant. This will make a little more sense when discussing the laws!
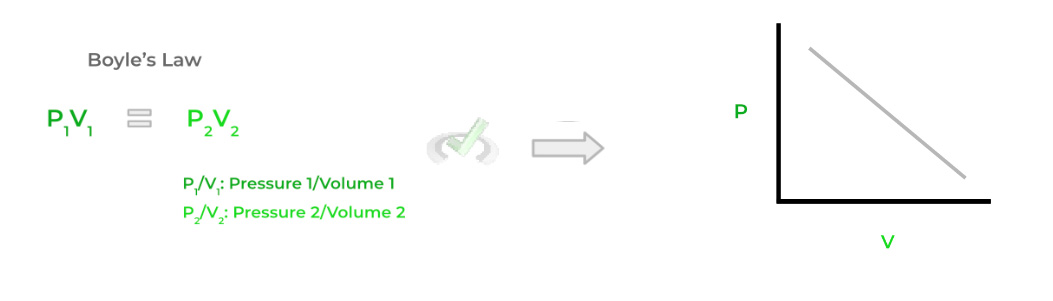
A. Boyle’s Law: Indirect/Inverse Relationship Between Pressure and Volume
(i.e. If Pressure Increases, Volume Decreases — and vice versa).
What Stays Constant?
- Temperature (T)
- Moles of Gas (n)
B. Charles’ Law: Direct Relationship Between Temperature and Volume
(i.e If Temperature Increases, Volume Increases — and vice versa).
What Stays Constant?
Pressure (P)
Moles of Gas (n)

C. Guy-Lussac’s Law: Direct Relationship Between Temperature and Pressure
(i.e. If Temperature Increases, Pressure Increases — and vice versa).
>> What Stays Constant?
Volume (V)
Moles of Gas (n)
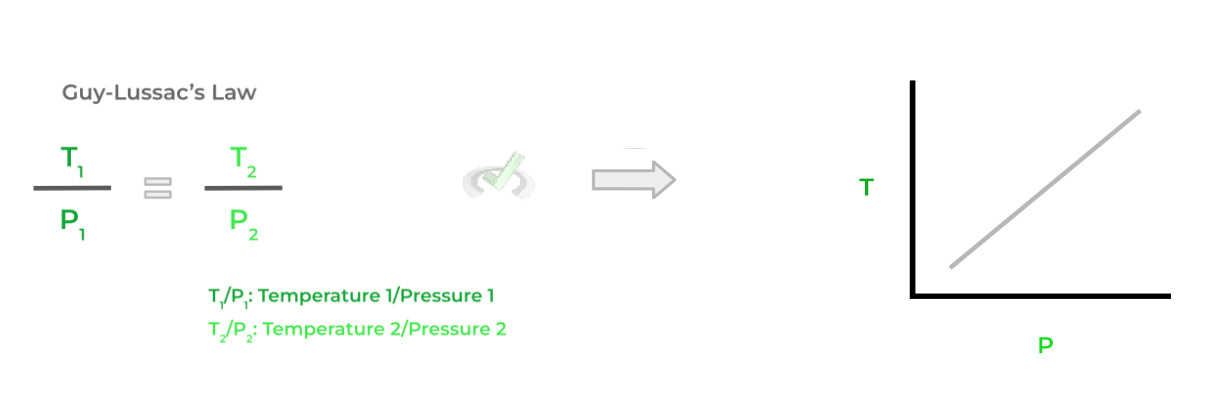
We also included a graph that gives a better visual representation of the relationship between the variables for a specific law! Those with a positive slope indicate a direct relationship while a negative slope indicates an indirect/inverse relationship.
(Coming Soon!) Full Study Notes : High Yield Gas Laws on the MCAT
For more in-depth content review on main equations and a couple more lower yield ones, check out these detailed lesson notes created by top MCAT scorers.
4. Kinetic Molecular Theory
Despite the big words that are used, this theory concerning gasses is actually relatively simple! You can actually think of this as an extension of our discussion of ideal gasses, just with an added emphasis on the movement of the particles!
There are 5 main principles for kinetic molecular theory. We’ve already discussed 2 of them as the main assumptions for ideal gasses:
1. There are no intermolecular interactions between the gas particles.
2. The gas particles themselves have negligible volumes and don’t take up any space.
The remaining assumptions deal more with the movement of the gas particles! We’ll cover the next 2 points together below as they are related to one another.
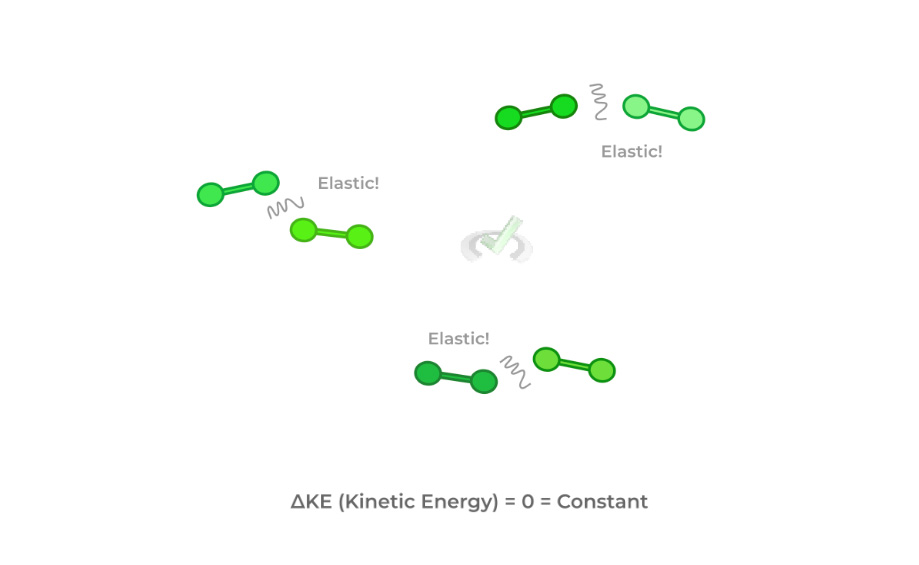
3. The motion of the gas particles within the container is continuous and random, colliding with other gas particles and the container.
4. The collisions of the gas particles with each other and the gas containers are elastic.
Pay special attention to the 4th assumption! The elastic collisions result in the overall kinetic energy of the particles remaining constant!
5. The average kinetic energy of the gas particle is determined by the temperature of the gas.
Finally, we also wanted to make one more distinction between temperature and thermal energy! Temperature refers to the average kinetic energy of the gas particles while thermal energy refers to the total kinetic energy!
(Coming Soon!) Full Study Notes : Kinetic Molecular Theory on the MCAT
For more in-depth content review on kinetic molecular theory, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about the gas phase in general chemistry!
Term | Definition |
|---|---|
Gas | Phase of matter characterized by no definite shape and volume |
Pressure | Defined as the ratio of the force of the gas particle and the surface area of the container |
Ideal Gas Law | Law which relates the variables of pressure (P), volume (V), moles (n), and temperature (T) of a gas all together |
Boyle’s Law | Gas law which states that there’s an inverse relationship between the pressure and volume of a gas, given constant moles and temperature |
Charles’ Law | Gas law which states that there’s a direct relationship between the temperature and volume of a gas, given constant moles and pressure |
Guy-Lussac’s Law | Gas law which states that there’s a direct relationship between the temperature and pressure of a gas, given constant moles and volume |
Additional FAQs - The Gas Phase on the MCAT
What is an Ideal Gas? – MCAT
Is the Ideal Gas Constant on the MCAT?
What are the 3 Gas Laws? – MCAT
Boyle’s law indicates an inverse relationship between pressure and volume. Charles’ law indicates a direct relationship between temperature and volume. Finally, Guy-Lussac’s law also indicates a direct relationship between temperature and pressure.
Additional Reading Links (Coming Soon!) – Study Notes for the Gas Phase on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- Acids and Bases on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























