It’s amazing to think that all the things in the world — from animals, phones, and ourselves — are all built off of these small atoms that we can’t even see! To quantify the number of atoms that make up our whole planet, we’d probably need to be at least a type III civilization (mind blown…)!
The atomic structure of atoms not only tells us what atoms are composed of and their organization but also lays the foundation for how atoms interact with each other to form more molecules which in turn react with one another to form this expansive universe!
After going over this chapter overview, you should have the basics about atomic structure in relation to how it’s covered in general chemistry. Let’s get started!
Atomic Structure on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try to expect around 2-4 questions that can appear on the MCAT covering stoichiometric topics, probably with a higher emphasis on calculation based questions!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Atomic Structure
A great way to approach the study of atomic structure is “build it brick by brick”. We say this because topics can get slightly more confusing and complicated when you get into electron configuration and different electron subshells.
We’ll definitely employ this motif in the way we’ll organize and teach these topics! We’ll start off with the basic and easier to understand fundamentals and, slowly, but surely, introduce the more complex ideas and content.
1. The Subatomic Particles of the Atom
It’s amazing to believe that as small as atoms are, there are even smaller particles that constitute the main components of atoms, aptly called subatomic particles! There are 3 main subatomic particles: protons, neutrons, and electrons.
They all primarily differ in size, charge, and location within the atom: protons and neutrons are located in the atom’s nucleus, the atom’s dense center. Furthermore, protons are positively charged while neutrons retain a neutral charge.
Contrarily, electrons are negatively charged and are much smaller in size and mass than protons and neutrons. Additionally, electrons are not in the nucleus but are actually located in an “electron cloud”, which encloses the nucleus.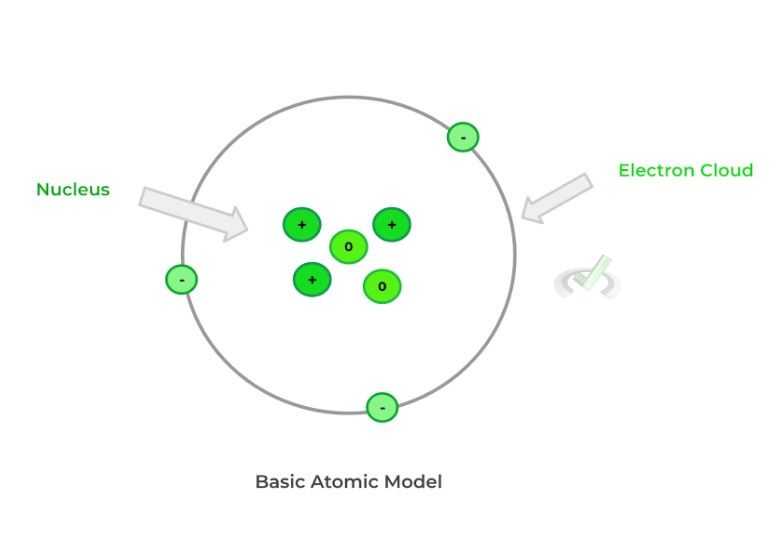
It’s important to note that changing the number of protons changes the elemental atom! For example, increasing the number of protons from 4 to 5 changes the elemental atom from beryllium to boron!
However, changing the number of neutrons or electrons doesn’t change the elemental atom, it just changes the type of isotope or ion, respectively! Check out the visual below for more detail and information!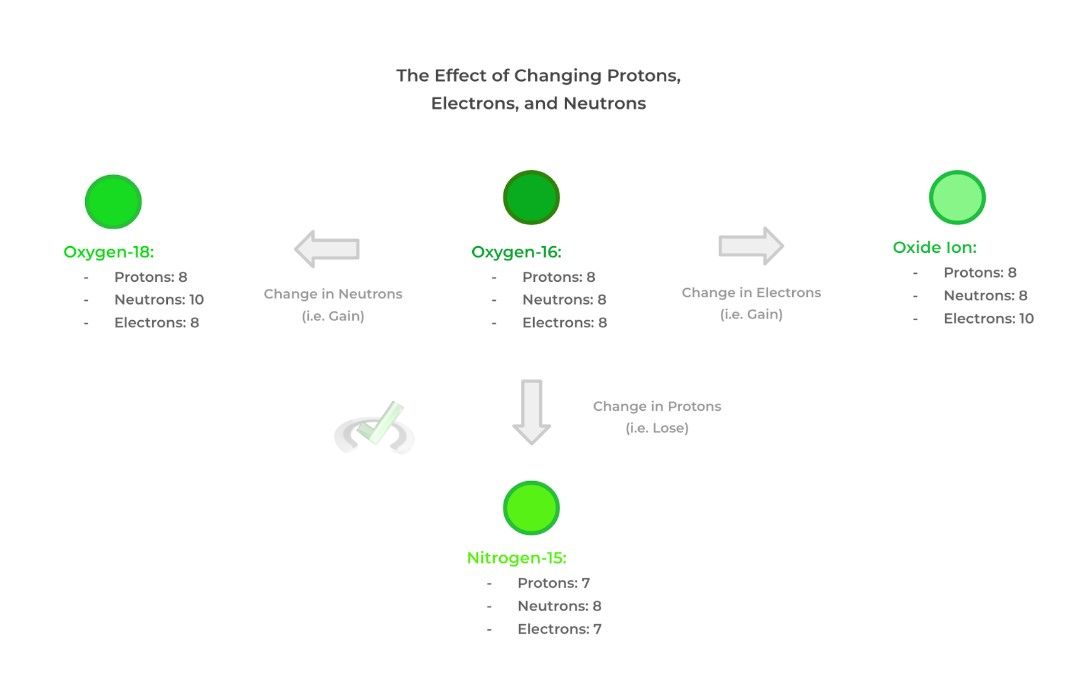
Additionally, note that a neutral atom is one where there’s an equal number of protons and electrons — it doesn’t necessarily need to have the same number of neutrons!
(Coming Soon!) Full Study Notes : The Subatomic Particles of the Atom
For more in-depth content review on the basic structure of atoms and their basic constituents, check out these detailed lesson notes created by top MCAT scorers.
2. Atomic Number v.s Mass Number v.s. Atomic Weight
It for sure can get a little confusing to differentiate between all these similar terms, but don’t worry! Similar to the advice we gave above, we’ll start from the most simply defined term and work our way up!
The atomic number simply refers to the number of protons within an elemental atom. The mass number is just a step up from atomic number, where we have to add both the amount of protons and neutrons.
As such, isotopes will have the same atomic number but will have different mass numbers due to the different amounts of neutrons. Look at the example below!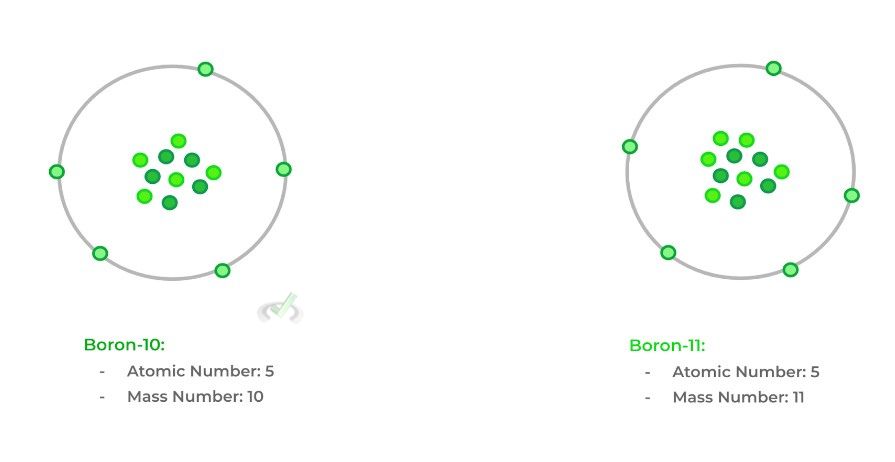
Finally, the atomic weight refers to the average mass of all the naturally occurring isotopes of an element. To help you remember this, always think of the term “weighted average” to aid in your review!
It should as be noted, however, that you must also take into account the percentages of the naturally occurring isotopes, as they’re not all equal! Sometimes, certain isotopes come up in nature more often than others and this uneven distribution must be taken into consideration!
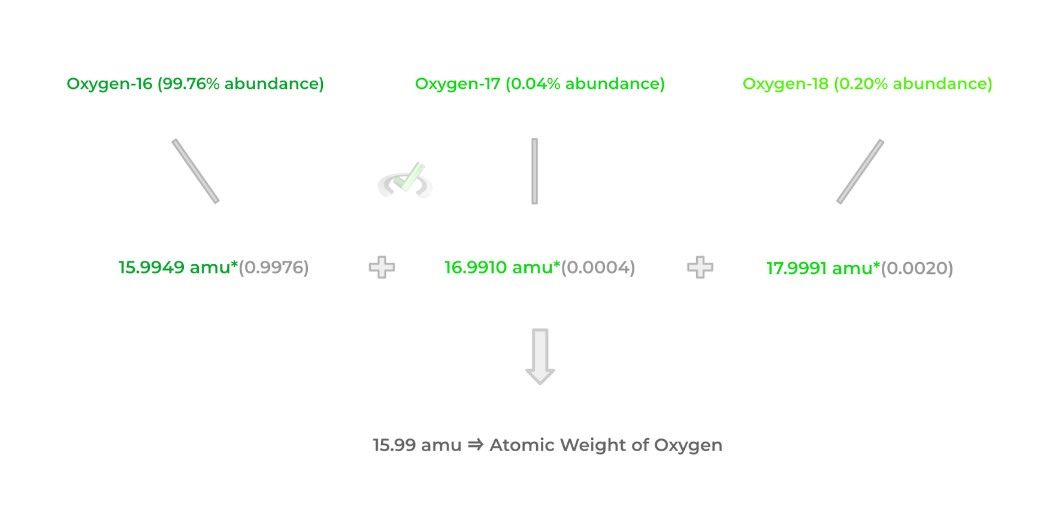
Click on this article for a more detailed coverage of the differences between all the terminology!
(Coming Soon!) Full Study Notes : Atomic Weight v.s. Atomic Mass; Atomic Number v.s. Mass Number
For more in-depth content review on atomic weight v.s. atomic mass; atomic number v.s. mass number, check out these detailed lesson notes created by top MCAT scorers.
3. The Bohr Model and Atomic Spectra
Let’s now move our attention away from the nucleus and focus more on the electrons as the electrons essentially guide the reactivity of atoms and how molecules react with one another during chemical reactions!
In order to first understand how electrons are organized in the electron cloud, it may be helpful to first use a simplified model called the Bohr Model. In this model, the electron cloud is represented by different principal shells, which are represented as circles — shells are where the electrons reside and travel.
One key idea to understand about the electron shells is that they differ in specific energy levels: each level is numbered numerically — 1, 2, 3, and so on — and uses the variable, n. The smaller the number is, the lower the energy state, while the higher the number is, the greater the energy state!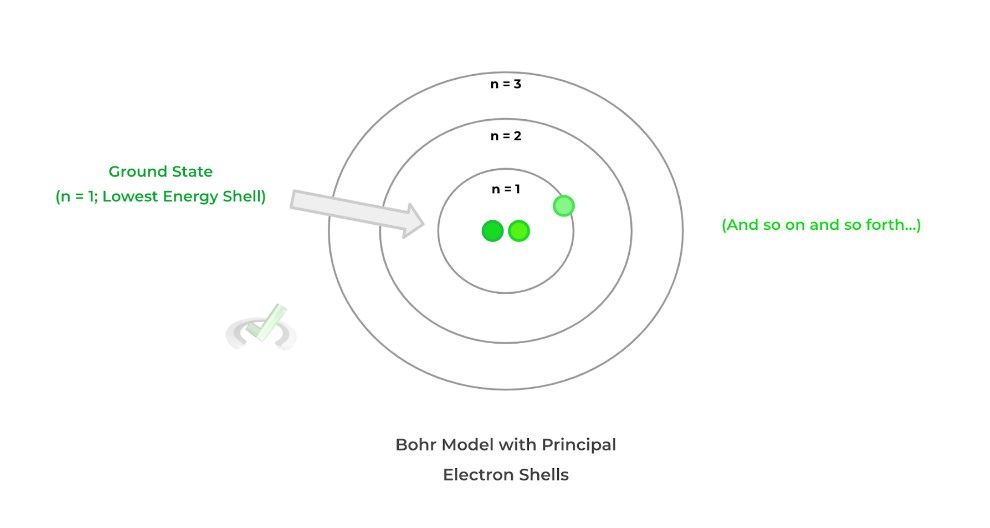
As mentioned above, the lowest energy shell (n =1 ) is referred to as the ground state because it’s where an electron is lowest in energy. This will come back when we discuss atomic emission and absorption!
Furthermore, as we move up the principal electron shells (n increases), the number of electrons that a shell can accommodate increases! For example, the first principal electron can hold only 2 electrons while the second can hold up to 8! To find how many electrons an electron shell can hold, use the equation below: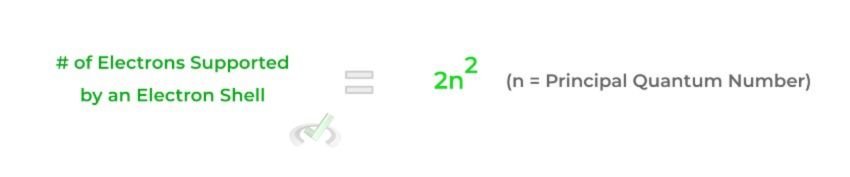
Finally, another important aspect to talk about which is easily visualized by the Bohr Model, is the atomic spectra. While electrons can only be located in one electron shell, they can also move between the shells!
In order to change between the electron shells, electrons must either gain or lose energy in the form of photons. Put in the simplest sense, photons are the basic units that makeup light, having a dual wave and particle nature.
As such, when photons are gained or released by an atom, light energy is absorbed or emitted — it’s this gain or release of energy that allows the electron to change energy levels! Look at the figure and relationship below: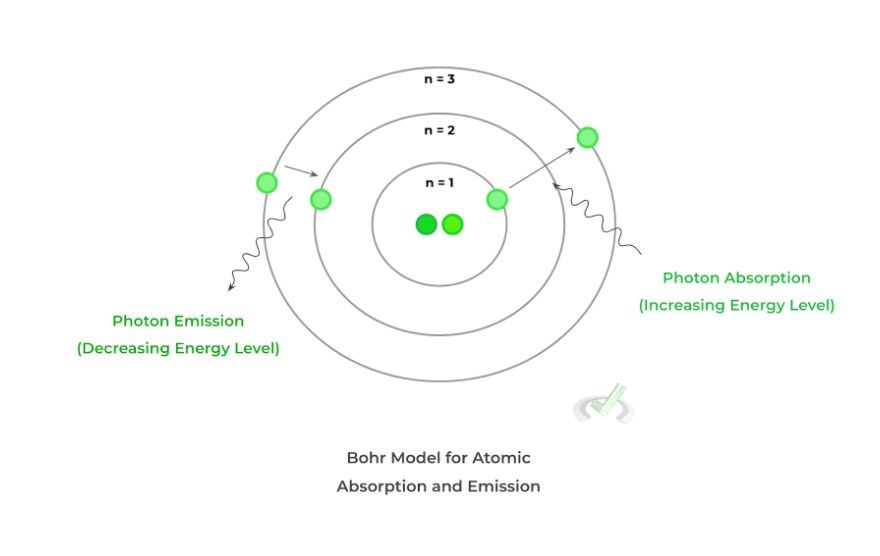
Though it has its flaws, the Bohr model is a great way to get introduced to the concepts of energy levels and atomic spectra.
(Coming Soon!) Full Study Notes : The Bohr Model and Atomic Spectra
For more in-depth content review on the Bohr model and atomic spectra, check out these detailed lesson notes created by top MCAT scorers.
4. Understanding Quantum Numbers
While this is for sure one of the more tricky and complicated topics covering atomic structure, we’ll break it down bit by bit! The reason we use quantum numbers is to describe electrons in terms of their most probable location in an atom’s electron cloud — think of them as kinda like an address!
As mentioned in the last article, electrons are located in one of the principal electron shells, also called the principal quantum number (n). Furthermore, principal shells can be broken down into subshells, called the azimuthal quantum number (l).
This number specifically determines the type of shape of the orbital an electron occupies. An orbital is simply a region of space where electrons are most likely located in the electron cloud. There are 4 main types of subshells that you’ll encounter: s, p, d, and f subshells.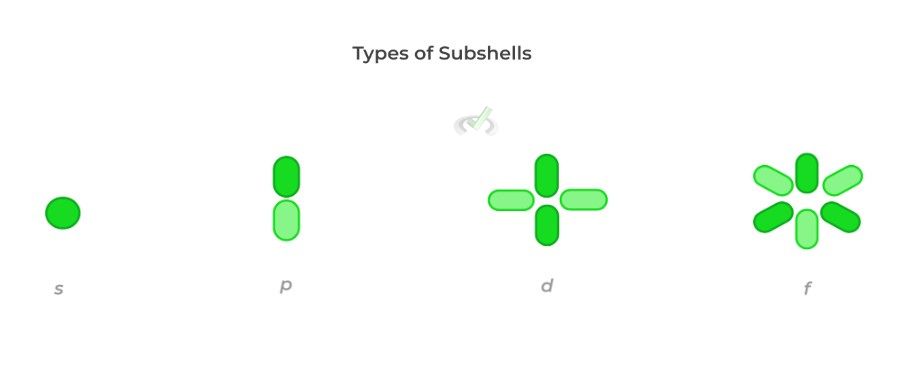
When you increase the principal quantum number, the number of subshells increases as well! We can use the equation below to see which subshells are present within a principal electron shell:

Let’s give an example utilizing the n = 2 principal quantum number: given the relationship above, the accepted values for the azimuthal quantum number (l) are 0 and 1 (because 2 - 1 = 1). Each (l) value corresponds to a specific subshell as shown below:

Going back to our example above, the n = 2 principal quantum number has 2 types of subshells: the s and p subshells.
The 3rd quantum number is called the magnetic quantum number (ml) and simply refers to the orientation of an orbital. Similarly, there’s also a relationship between the azimuthal quantum number (l) and the magnetic quantum number (ml) shown below: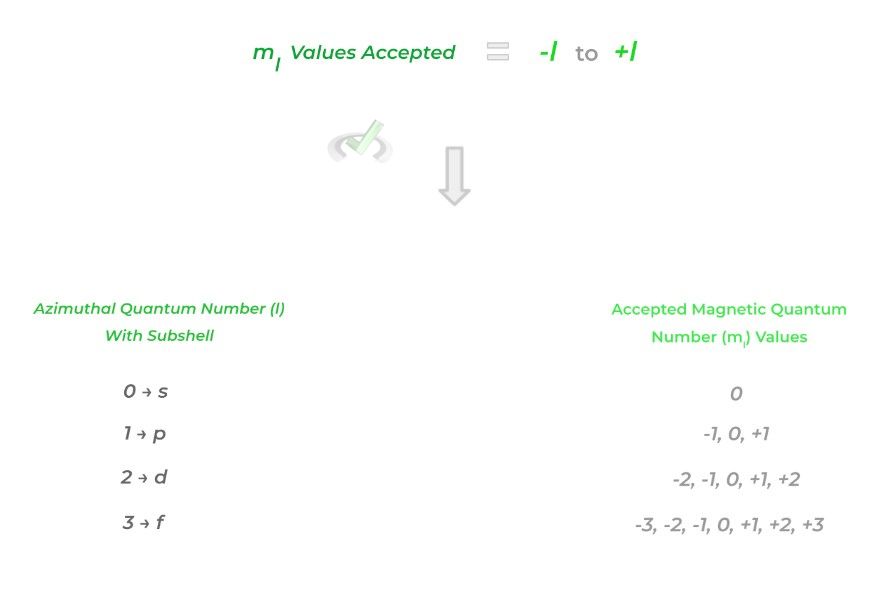
Additionally, this value also can tell you how many orbitals a specific subshell has. Take the p subshell. For example, the p subshell (l = 1) has 3 possible magnetic quantum numbers (ml): -1, 0, and +1. Each of these 3 magnetic quantum numbers represents 3 different orbitals, each differing with orientations!
The final (and probably simplest) quantum number is the electron spin quantum number (ms) and simply describes the orientation of an electron’s spin: clockwise or counterclockwise.
Because the spin of an electron can only be clockwise or counterclockwise, there are only 2 values that can be given for the electron spin, +½ and -½! This rule is highly important because orbitals can only hold up to 2 electrons — this explains why there are only 2 values for the electron spin quantum number!
Though it can be tricky, understanding quantum numbers takes a little time to grasp; however, we also encourage you not to get so focused and engulfed with understanding all the specifics!
(Coming Soon!) Full Study Notes : Understanding Quantum Numbers
For more in-depth content review on quantum numbers and how to approach them, check out these detailed lesson notes created by top MCAT scorers.
5. Rules of Electron Configuration
We wanted to cover the topic of quantum numbers first because they lay the foundation for the electron configuration of elemental atoms! Simply put, electron configuration describes how electrons are distributed within the orbitals of the electron cloud.
There are 3 main rules we’ll cover briefly for the scope of this chapter overview: the Aufbau principle, the Pauli Exclusion principle, and Hund’s rule. These 3 rules are without a doubt high yield and should be understood come test day!A. Aufbau Principle
To understand this principle, it’s important to grasp that the different principal electron shells, as well as their subshells have different energy levels.
Aufbau principle states that when assigning electrons to the orbitals, we have to fill the orbitals from the lowest to highest energy level! If you've taken some general chemistry classes, you’re probably familiar with the diagram below: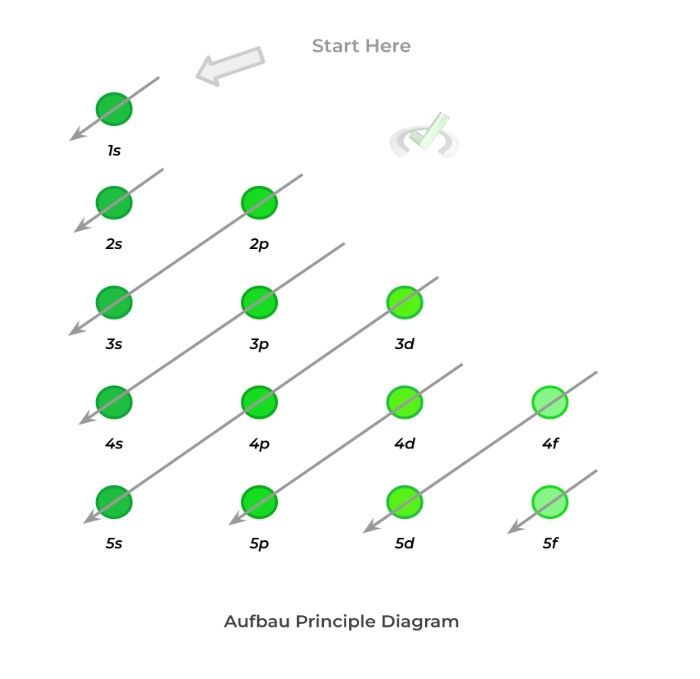
As shown above, electrons must occupy orbitals from a lower energy level before occupying those at higher energy levels! You can use the arrow trick shown in the diagrams so that you don’t have to memorize the exact order!
B. Pauli Exclusion Principle
The best way to think about this principle is similar to a cell phone number or social security number: no 2 electrons can share the same set of quantum numbers.
Recall that the quantum numbers are used to describe the most likely location of an electron. This rule is a great segway into how we organize electrons when displaying the electron configuration of elemental atoms.
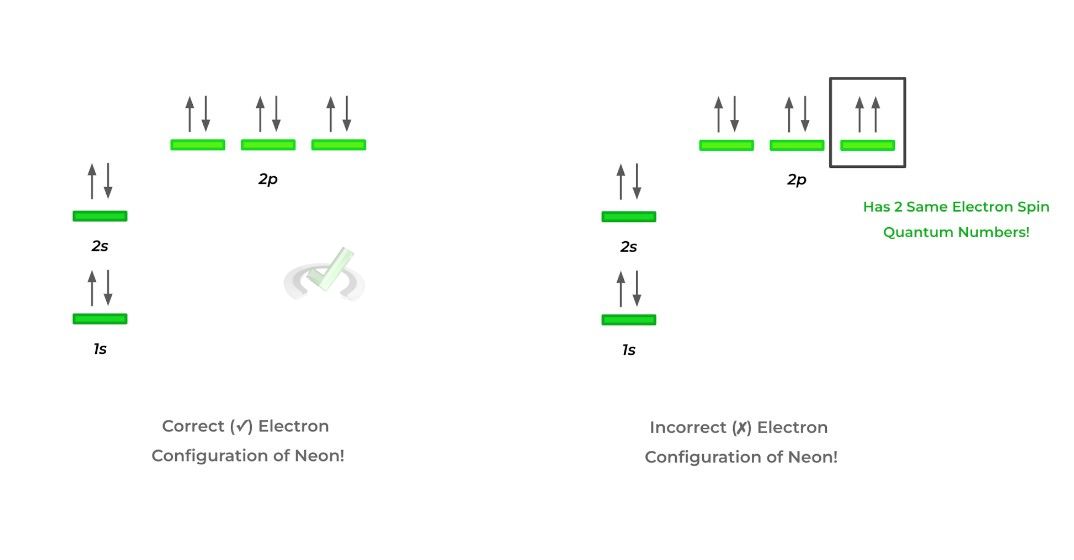
As shown above, we organize the orbitals to ascending energy levels in order to follow Aufbau’s principle. Likewise, notice also how each orbital only contains 2 electrons represented by 2 arrows: one pointing up (clockwise spin) and one pointing down (counterclockwise spin).
The electron configuration of neon (10 electrons) on the right side breaks the Pauli exclusion principle because the 2 box electrons have 2 identical sets of quantum numbers — this is shown by the 2 electrons having the same electron spin!
C. Hund’s Rule
Finally, Hund’s rule states that when assigning electrons to a subshell, they must occupy an empty orbital before pairing up. This is probably better understood when given a visual, as shown below!
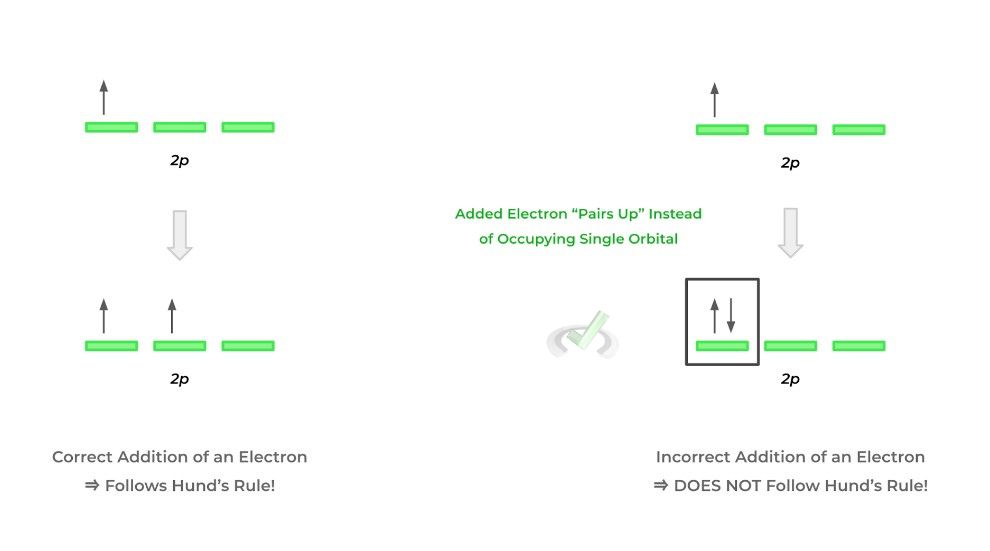
These are some of the basic rules which serve as the foundations for how to construct the electron configurations of elemental atoms!
(Coming Soon!) Full Study Notes : Rules of Electronic Configuration
For more in-depth content review on the rules of electronic configuration, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about atomic structure in general chemistry!
Term | Definition |
|---|---|
Protons | The subatomic particle which is located in the nucleus and is positively charged |
Neutrons | The subatomic particle which is located in the nucleus and has a neutral charge |
Electrons | The subatomic particle located in the electron cloud and is negatively charged |
Ions | Refers to the same elemental atoms which differs in the number of electrons |
Isotopes | Refers to the same elemental atoms which differs in the number of neutrons |
Atomic Number | Equivalent to the number of protons in an elemental atom |
Mass Number | Equivalent to the sum of the protons and neutrons in an elemental atom |
Atomic Weight | Refers to the average mass of an element’s naturally occurring isotopes |
Photons | The main units of light which as a dual particle-wave characteristics |
Absorption |
The gain of energy from photons which results in an electron increasing in an energy level |
Emission |
The loss of energy via the release of photons which results in an electron decreasing in an energy level |
Principal Quantum Number (n) |
Quantum number which gives the main electron shell and energy level |
Azimuthal Quantum Number (l) |
Quantum number which determines the shape of the subshell |
Magnetic Quantum Number (ml) |
Quantum number which determines the orientation of the subshell |
Electron Spin Quantum Number (ms) |
Quantum number which determines the spin of an electron in an orbital |
Aufbau’s Principle |
States that when filling orbitals, electrons must first occupy lower energy orbitals before occupying higher energy ones |
Pauli Exclusion Principle |
States that no electrons can share the same set of quantum numbers |
Hund’s Rule |
States that electrons must first fill in free orbitals before “pairing up” with other electrons |
Additional FAQs - Atomic Structure On the MCAT
Is General Chemistry I on the MCAT?
Do You Need to Know the Periodic Table for the MCAT?
What is the Bohr Model? – MCAT
What Are the 4 Quantum Numbers? – MCAT
Additional Reading Links (Coming Soon!) – Study Notes for Atomic Structure on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Acids and Bases on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these