Oftentimes in our daily lives, we want to get to our goals and desires as fast as possible. Probably the most relatable example is in all of us where we all want to get to medical school and become a physician ASAP: however, as with all things in life, most things are slow and take time to achieve, step by step.
The parallel with chemical reactions is uncanny: in order for a chemical reaction to progress and eventually form the products, they must undergo certain steps in order to reach the resulting outcome!
This chapter overview should give a basic overview of chemical kinetics including the various theories, mechanisms, and equations related to the topic. Let’s go!
Chemical Kinetics on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Here at MCAT Mastery, we calculated around a 5-7 question average that will cover chemical kinetics. These don’t necessarily have to be asked in the context of general chemistry as these questions can also have applications in organic and biochemistry!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Chemical Kinetics
Try to study and review this material in conjunction with similarly related topics such as enzymes, thermodynamics, and equilibrium!
If you can study these topics and chapters within the same time frame, such as within the same week or two, you’ll see the overlap present and will have a better chance of internalizing the content reviewed as there’s significant overlap!
1. Basics of Reaction Mechanisms
Before getting into the fundamentals of reaction mechanisms, it’s key to differentiate the difference between kinetics and spontaneity. Kinetics refers to how fast a reaction will occur while spontaneity — a thermodynamic property — refers to how likely a reaction will occur.
As mentioned above, a chemical reaction can’t simply have the reactant particles collide to form the product (although this does have some merit). In actuality, chemical reactions must follow a reaction mechanism.
A reaction mechanism is a series of steps that reactants must take in order to form the final product(s). A reaction mechanism must have a minimum of 2 steps — one slow and one fast step — and must summate to the overall reaction as shown below!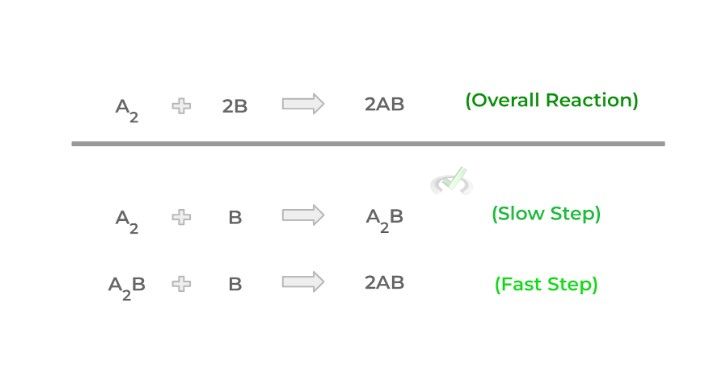
Though there are two steps as shown above, really the only important one to pay attention to is the slow step which is also known as the rate determining step. As its name implies, how fast a reaction will occur depends on this step. It’s basically analogous to the limiting reagent in stoichiometry problems!
As we’ll see later as well, this step will be the only one considered when determining the rate law of a reaction which is basically an equation we can use to calculate the reaction rate (i.e. how fast a reaction will occur).
(Coming Soon!) Full Study Notes : Basics of Reaction Mechanisms
For more in-depth content review on reaction mechanism basics, check out these detailed lesson notes created by top MCAT scorers.
2. Theories and Factors Affecting Chemical Kinetics
Within chemical kinetics, there are 2 main factors that are used to explain how chemical reactions progress: 1) collision theory and 2) transition state theory. Though the latter is really more accurate in terms of a theoretical approach, a synthesis of both is necessary to completely explain chemical kinetics!
Collision theory basically states that when reactant particles collide with each with enough energy, the product(s) — this minimum amount of required energy is called the activation energy (Ea).
There are 2 main ways we can increase the collisions of the reactant particles and increase the likelihood that the energy is equal to or exceeds Ea: increasing the temperature and increasing the reactant concentration.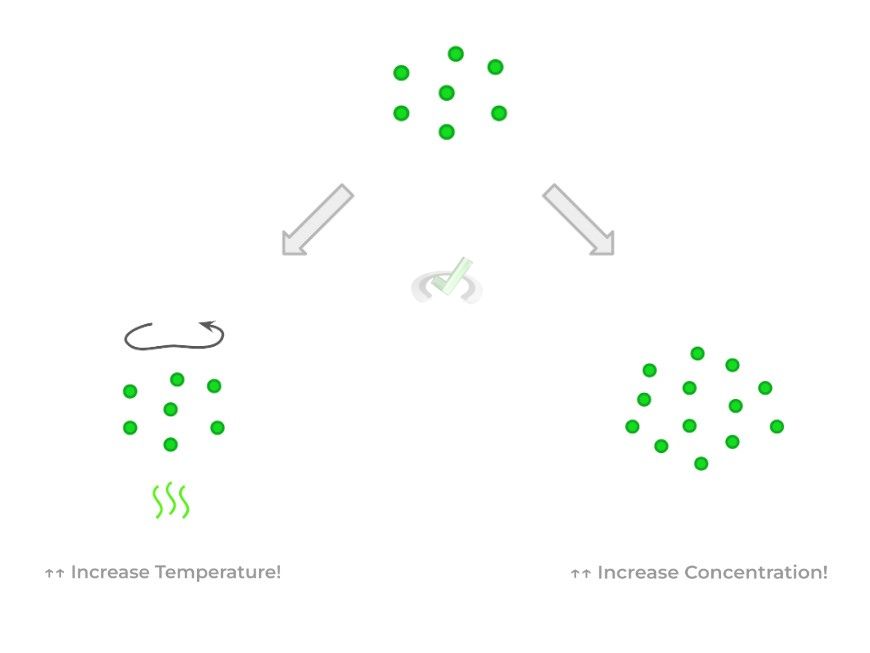
While this is a good start for explaining chemical kinetics, the transition state theory is viewed more favorably by chemists as it better explains the thermodynamics that is also in play!
As stated by its name, the main component of this theory is the idea of a transition state which is a high energy molecular configuration along the reaction coordinate of a chemical reaction. This may be a little hard when put into words and is better understood via a visual!
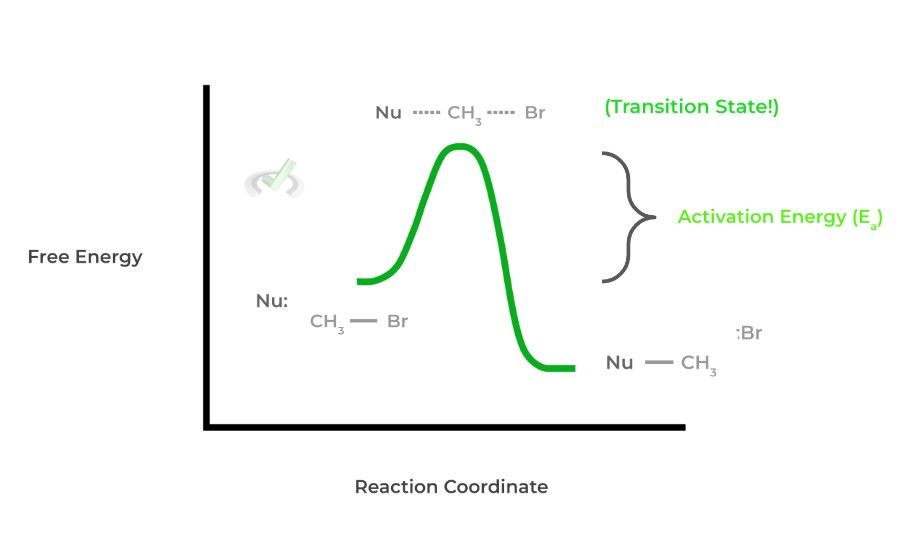
There are a couple of important takeaways from this: notice how the difference in energy levels between the reactants and the transition state is equal to the activation energy. In this way, we still have an application of collision theory.
Likewise, when viewed this way, notice how also the transition state can either form either the reactant or product. This is important when considering equilibrium as now we can also see how reversible reactions relate to chemical kinetics!
(Coming Soon!) Full Study Notes : Theories and Factors Affecting Chemical Kinetics
For more in-depth content review on the different factors and theories that are associated with chemical kinetics, check out these detailed lesson notes created by top MCAT scorers.
3. Understanding Reaction Rate and Rate Laws
In general, when the word reaction rate comes to mind, it’s fair to say that it simply refers to how fast a chemical reaction takes place! For a more precise definition, we can actually define the reaction rate as how fast the reactant(s) disappears and/or how fast the product(s) appears.
This should make sense as during the course of a chemical reaction, the reactants will disappear as they react together to form products. We can actually model this as an
equation given the chemical equation below:
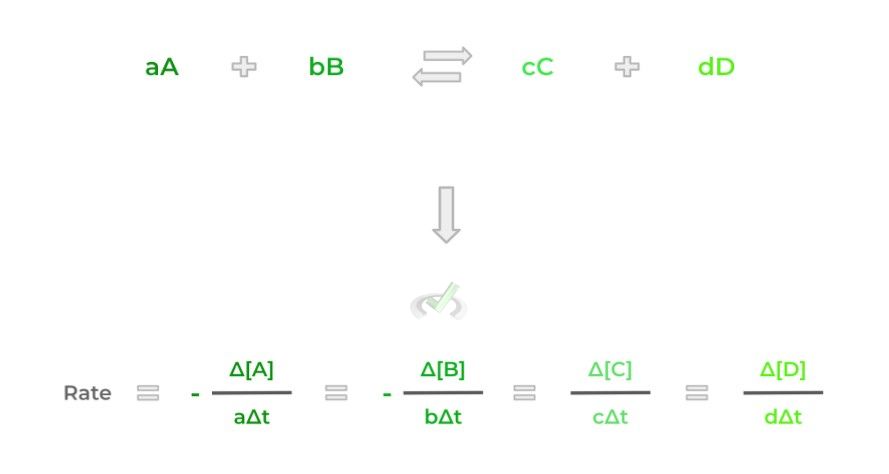
There are a couple of things to note about the equation: notice how there’s a negative sign in front of reactants A and B — this is to indicate that the reactants are disappearing in order to form products.
Additionally, notice also how the concentrations of the reactants and products are divided by their stoichiometric coefficients. This is to ensure that there’s an equal standard to compare the rate of appearance/disappearance.
In addition to the equation above, another way to calculate the rate of a reaction is via the rate law. The rate law is an equation/expression where the reaction rate is equal to the product of a rate constant (k) and the concentration of the reactants raised to a certain power, as shown below.
You might notice that this equation looks pretty similar to the equation for the equilibrium constant. However, there is one important difference: the power of the reactants must be determined experimentally!
The powers 9 times out of 10 won’t be the same as the stoichiometric coefficients. You must actually measure the reaction rates with varying concentrations. Afterward, you see how changing the concentration affects the reaction rate. You’ll often see it displayed on a table as shown below:
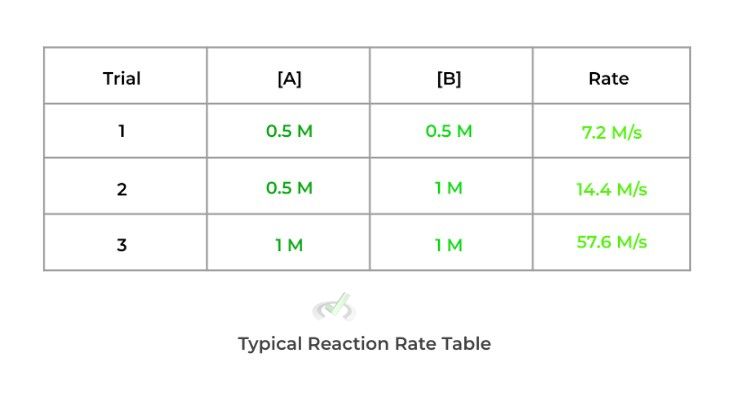
In order to find the power, more specifically called the order, follow the steps shown below:
a. To find the power of reactant A, find 2 trials where reactant B remains constant and reactant A.
b. Then, compare how changing [A] affects the reaction rate! Below are some of the main relationships to look out for.
[A] Doubles ⇒ Rate Remains Constant: Zero Order (x = 0)
[A] Doubles ⇒ Rate Doubles: First Order (x = 1)
[A] Doubles ⇒ Rate Quadruples: Second Order (x = 2)
c. Do the same for reactant B (and for any other reactants).
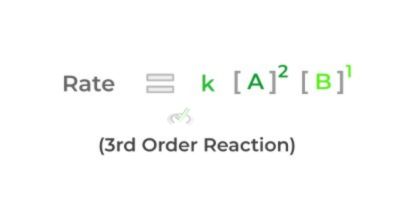
Using the example above, we see that doubling reactant A (while keeping reactant B) constant) quadruples the reaction rate making it a second order. Likewise, when we double reactant B (while keeping reactant A constant), the reaction rate also doubles, making it a first order.
To find the total order of the rate law, simply add the exponents together. Shown below is the completed rate law!
Finally, another important note to mention is that the rate constant varies with temperature. While of course the value remains constant at a certain temperature, the rate constant changes as you change the temperature.
(Coming Soon!) Full Study Notes : Understanding Reaction Rate and Rate Laws
For more in-depth content review on reaction rate and rate laws within chemical kinetics, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about chemical kinetics in general chemistry!
Term | Definition |
|---|---|
Reaction Mechanism | The series of steps reactants must take in order to form a product; Must have a minimum of 2 steps, with one being a slow step and one being a fast step |
Rate Determining Step | Also known as the slow step of the reaction mechanism; The reaction only goes as fast as the rate determining step |
Collision Theory | A theory describing chemical kinetics where reactants undergo constant, random collisions which allows them to form products |
Activation Energy | The minimum amount of energy required in order for a chemical reaction to take place |
Transition State Theory | Another theory describing chemical kinetics where a high energy configuration, called the transition state, is a main component; The energy difference between the reactants and the transition state is equal to the activation energy |
Reaction Rate | Defined as both the rate of disappearance of the reactants and/or the rate of appearance of the products |
Rate Law | An equation/expression where the reaction rate is equal to the product of a rate constant and the concentration of the reactants raised to a certain power |
Additional FAQs - Chemical Kinetics on the MCAT
Are Chemical Kinetics on the MCAT?
What are the Topics Covered in Chemical Kinetics? – MCAT
How are Chemical Kinetics Used in Medicine? – MCAT
Additional Reading Links (Coming Soon!) – Study Notes for Chemical Kinetics on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Acids and Bases on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























