Your MCAT preparation journey shouldn’t be complete without answering practice questions.
Practice questions are a great way to know where you are in your MCAT preparation. At the same time, they also help you identify your strong and weak areas to focus more on and which topics you need to improve on.
This article is here to give a few MCAT organic practice questions.
If you want to know how you are doing in your MCAT preparation and want to identify the concepts you are weak at, please have your pen and paper ready. Let’s keep going!
What is MCAT Organic Chemistry?
Organic chemistry studies the structures, properties, and interactions of organic compounds, which have covalent bonds between carbon atoms.
Chemical reactivity is evaluated in organic chemistry along with physical and chemical factors to understand behavior.
The MCAT's Chemical and Physical Foundations of Biological Systems and Biological and Biochemical Foundations of Living Systems sections both cover organic chemistry.
Organic chemistry covers 15% of the Chem/Phys component of the MCAT. This means that 9 of the 59 questions in this area will demand your knowledge of and skills in organic chemistry.
Additionally, organic chemistry is covered in 5% of the MCAT's Bio/Biochem section. This means that there are 3 questions (out of 59) that are about MCAT organic chemistry in this section.
Summary Table of Organic Chemistry Distribution in the MCAT
MCAT Section | Chemistry Subject | Percentage | Number of Questions (out of 59) |
|---|---|---|---|
Chemical and Physical Foundations of Biological Systems | Organic Chemistry | 15% | 9 |
Biological and Biochemical Foundations of Living Systems | Organic Chemistry | 5% | 3 |
Total Number of MCAT Organic Chemistry Questions: 12 | |||
Organic Chemistry Topics to Study for the MCAT
To best prepare for the MCAT chemistry, you must be familiar with the broad variety of topics covered in MCAT organic chemistry. Exert enough effort and time studying these topics to achieve optimum results.
The different topics covered in the MCAT organic chemistry are
MCAT Organic Chemistry Practice Questions
As mentioned earlier, you need to answer practice questions during your MCAT preparation. Those who have taken the MCAT would agree that practicing test questions before the exam day prepared and gave them the confidence they needed for the MCAT.
By taking practice exams, you can get accustomed to the questions' structure and the variety of possible responses. Additionally, they evaluate your preparation level and offer assistance as needed.
So, have paper and pencil handy and get ready to answer the MCAT organic chemistry questions below:
1. What results when water and pentyl ethanoate are combined in an acidic environment?.
A. Water and butanol
B. Pentanol and ethanoic acid
C. Two equivalents of ethanoic acid
D. Two equivalents of pentanol
2. To remove isobutyric acid from the solution, diethyl ether solution should be washed with:
A. Hexane wash of 30 mL
B. One wash of 30 mL of water
C. Three 10 mL water rinses
D. Three hexane washes of 10 mL each
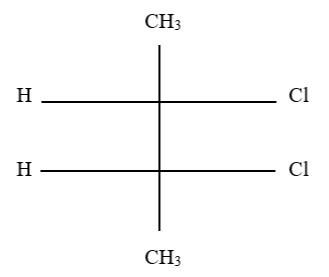
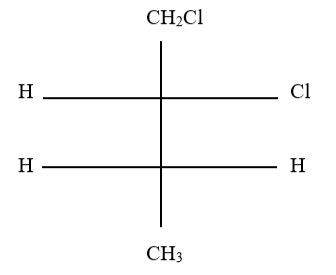
3. What would the name of the parent root of the molecule below be if all prefixes were removed?
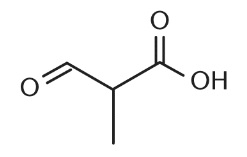
A. Propanol
B. Propanoate
C. Propanoic acid
D. Anhydride of propionate
4. What prefix comes first in the popular names of the one-carbon aldehydes and carboxylic acids?
A. Acet-
B. Form-
C. Meth-
D. Para-
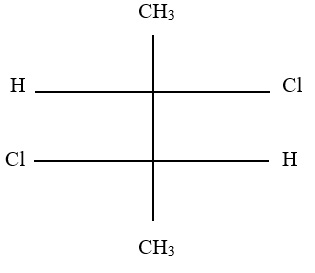
5. What is the number of stereoisomers for the following aldehyde?
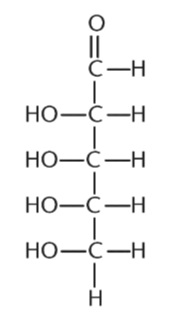
A. 2
B. 8
C. 10
D. 16
6. A proton pump inhibitor frequently prescribed for gastroesophageal reflux disease is omeprazole. Pharmaceutical companies started producing esomeprazole, the (S)-enantiomer of omeprazole, on its own after the racemic mixture form of omeprazole lost its patent protection. Which of the omeprazole and esomeprazole 1 M solutions is more likely to have optical activity?
A. Only omeprazole is prescribed.
B. Only esomeprazole is prescribed.
C. Both esomeprazole and omeprazole are prescribed.
D. Neither of the two is prescribed.
7. Take into account (E)-2 butene and (Z)-2 butene. Which class(es) of isomers comprise this pair?
I. Cis-trans isomers
II. Diastereomers
III. Enantiomers
A. I only
B. II only
C. I and II only
D. I and III only
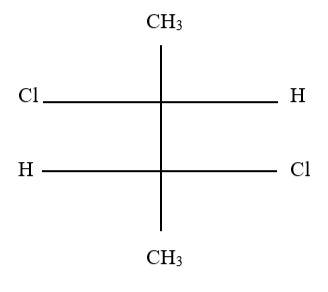
8. Which of the following substances has minimal optical activity?
9. The carbon and nitrogen atoms in CN- have the following hybridizations:
A. sp3 and sp3, respectively
B. sp3 and sp, respectively
C. sp and sp3, respectively
D. sp and sp, respectively
10. Rank amine, carboxylic acid, aldehyde, and alkane in decreasing order of oxidation state.
A. Aldehyde, amine, alkane, carboxylic acid
B. Carboxylic acid, aldehyde, amine, alkane
C. Carboxylic acid, amine, aldehyde, alkane
D. Alkane, amine, aldehyde, carboxylic acid
11. Why does the equilibrium between enol and keto tautomers lean heavily toward enol?
I. The keto form has greater thermodynamic stability.
II. Enol is a lower energy form.
III. The enol form has a higher thermodynamic stability.
A. I only
B. III only
C. I and II only
D. II and III only
12. The phosphoric acid hydrogens have pKa values that:
A. prohibit buffering.
B. permit modest buffering across a narrow pH range.
C. permit a moderate capacity for buffering over a wide pH range.
D. high buffering capability across a narrow pH range is permitted.
13. In the image below, a scientist is doing the Strecker synthesis to create valine. Which of the following substances that contain carbonyl would be a suitable beginning reactant in this synthesis?
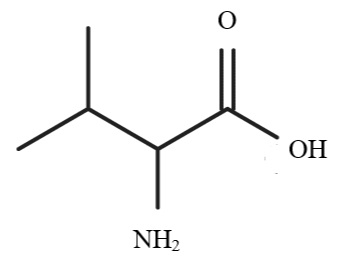
A. Butanal
B. Propanal
C. 2-Propanone
D. 2-Methylpropanal
14. In an aqueous solution, pyrophosphate is likely to:
A. have stability and inertia
B. produce insoluble compounds
C. make the solvent's polarity less intense
D. decompose to form inorganic phosphate
15. Which among the following could react with a carbonyl carbon to create a geminal diol?
A. Water
B. Ethanol
C. Hydrogen peroxide
D. Potassium dichromate
MCAT Organic Chemistry Sample Passage
The architecture of molecules is not rigid and constant. Even in relation to one another, their atoms are constantly bouncing around the average lengths and angles of their bonds. For instance, there are three potential vibrational modes in non-linear triatomic molecules: symmetric stretch mode, asymmetric stretch mode, and bending mode. Symmetric stretch occurs when the molecule's two bonds simultaneously extend and contract while asymmetric stretch occurs when one bond lengthens while the other bond compresses. The bending mode is where the bond angle can alternately widen or narrow.
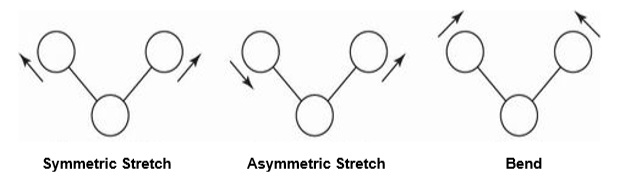
Figure 1 Vibrations of a Triatomic Molecule
A molecule's atoms can move in three different directions, commonly denoted by x, y, and z, in general. With N atoms in a molecule, 3N different atomic movements are allowed. But vibrational movement rather than translational movement will ensue if all the atoms in a molecule move in the same direction. Similar to how some atomic motion combinations can cause a molecule to rotate rather than vibrate, there exist these motion combinations as well. Accordingly, a molecule with N atoms will have 3N - 6 normal vibration modes in non-linear molecules and 3N - 5 normal vibration modes in linear molecules.
If we roughly assume that the atoms in a molecule are harmonic oscillators, then the vibrational energy of those atoms can be calculated as follows:
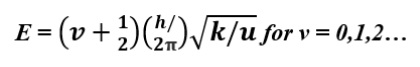
where k is the force constant of the bond, which rises with bond strength, v is the quantum vibrational number, h is Planck's constant, and u is the molecule's decreased mass. Energy changes in the vibrational quantum state are connected to infrared photon-like energies. Consequently, IR spectroscopy is the investigation of the energetics of the quantum vibrational states of molecules. However, IR light can only stimulate those common modes of vibration that cause a change in a molecule's dipole moment.
Molecule | Bond Energy (kJ/mol) |
|---|---|
H2 | 436 |
N2 | 946 |
O2 | 497 |
F2 | 155 |
16. According to VSEPR theory, the trigonal bipyramidal geometric family contains T-shaped molecules, each of which has exactly three atoms attached to it and two lone pairs of electrons. Which of the subsequent molecules has the shape of a T?
A. SF4
B. NH3
C. FO3-
D. BrCl3
17. Which of the following changes in molecular mobility can NEVER result in an IR spectra peak?
A. Bending and rotation
B. Bending and stretching
C. Rotation and translation
D. Translation and vibration
18. Which among the following molecules will have the highest vibration energy in the v = 0 state, if their decreased masses are the same?
A. N2
B. O2
C. F2
D. Cannot be determined based on the provided information
19. The formula for calculating the decreased mass of a diatomic molecule is u = (m1 * m2) / (m1 + m2), where m1 and m2 represent the two bound atoms' atomic weights. If the force constant k is the same for both D2 and H2, what will be the ratio (D2 to H2) of their ground state vibration energies?
A. 0.3
B. 0.7
C. 1.4
D. 2.1
20. In an IR spectrum, each of the following compounds will show absorption peaks EXCEPT:
A. O2
B. SO3
C. CO
D. HClO4
Answer Key:


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 




















