Most MCAT test-takers would agree that one of the best methods to ace the MCAT exam is to answer practice questions.
Why? By responding to MCAT practice questions, you become familiar with the MCAT's question format. They also assist you in determining your level of MCAT preparedness and the areas that you need to study more on.
The MCAT covers a lot of subjects, such as chemistry, biology, physics, biochemistry, psychology, and sociology. As you gear up for the MCAT, ensure that you take enough practice tests that cover all these subjects.
This article is here to share with you a few MCAT general chemistry practice questions. Have your pen and paper ready, and let’s start!
What is MCAT General Chemistry?
General Chemistry, commonly referred to as "gen chem," is a foundational level chemistry course that is offered by colleges and universities and is typically taken by first-year students.
The study of matter, energy, and their interactions is known as general chemistry. Some of the key subjects in general chemistry include bases and acids, the periodic table, atomic structure, chemical processes, and chemical bonding.
Both MCAT sections, Biological and Biochemical Foundations of Living Systems and Chemical and Physical Foundations of Biological Systems, include general chemistry questions.
Your knowledge and abilities in general chemistry are required for thirty percent (30%) of the MCAT Chem/Phys section. This indicates that 18 of the 59 questions on the MCAT are related to general chemistry.
On the other hand, 5% of general chemistry is included in the MCAT's bio/biochem part. Hence, this part contains 3 questions that concentrate on MCAT general chemistry.
Summary Table of General Chemistry Distribution in the MCAT
MCAT Section | Chemistry Subject | Percentage | Number of Questions (out of 59) |
|---|---|---|---|
Biological and Biochemical Foundations of Living Systems | General Chemistry | 30% | 18 |
Chemical and Physical Foundations of Biological Systems | General Chemistry | 5% | 3 |
Total Number of MCAT General Chemistry Questions: 21 | |||
General Chemistry Topics to Study for the MCAT
To properly prepare for the MCAT chemistry, you must be conversant with the many topics included in MCAT general chemistry. Spend enough time studying these topics to feel confident before taking the MCAT.
The list below shows the different concepts covered in the MCAT general chemistry:
MCAT General Chemistry Practice Questions
Nobody would argue against the idea that answering practice questions before the actual MCAT is helpful for test takers.
You can become used to the format of the questions and the range of answers by taking practice exams. In addition, they assess your level of readiness concurrently and provide help where it is required.
Here are a few MCAT general chemistry practice questions for you to answer, so have paper and pencil ready.
1. Which of the following activities DOES NOT change a reaction's equilibrium state?
A. Increasing or decreasing heat.
B. Changing the amount of a catalyst.
C. Altering the volumes of the reactants.
D. Altering the concentrations of the reactants.
2. Which of the following must be TRUE in order for a reaction to take place?
I. The particles of the reactant must clash.
II. Correct orientation of the reactant particles is necessary.
III. Particles of the reactant have (sufficient energy in collisions)
A. I and II only
B. I and III only
C. II and III only
D. I, II, and III
3. Take into account the following energy diagram for the following question:
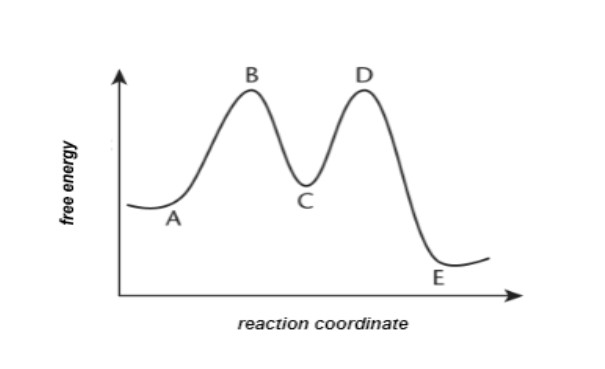
Which method has the greatest activation energy?
A. The initial step of the forward reaction
B. The initial step of the opposite reaction
C. The second step of the forward reaction
D. The second step of the opposite reaction
4. When the concentration of the first reactant is doubled in a third-order reaction with two reactants and two products, the rate rises by a factor of two. What will happen to the reaction rate if the second reactant's concentration is cut in half?
A. There will be a two-fold rise.
B. There will be a four-fold increase.
C. The amount will fall by a factor of 2.
D. The amount will fall by a factor of 4.
5. The energy of a photon is determined by the researchers to be 3.3 x 10-12J during an experimental trial. What is the wave's frequency?
(Note: The value of Planck's constant is 6.6 x 10-34)
A. 2.0 x 1022
B. 1.5 x 1022
C. 1.0 x 1022
D. 0.5 x 1022
6. Heat is applied to a sample of nitrogen gas inside a tight, rigid container. Because of the additional energy's effects, the pressure inside the container rises.
A. The gas molecules group themselves into clusters with more momentum.
B. Some nitrogen molecules split, increasing the pressure by creating extra particles.
C. As gas molecules travel more quickly, intermolecular collisions happen more frequently.
D. As the gas molecules move more quickly, there will be more collisions with the container.
7. At 20°C, MgSO4 dissolves in water at a rate of roughly 25 g/100 mL. A 0.25 g/mL solution of MgSO4 created at 37°C will have the following advantages over one made at 20°C:
A. Dissolve more slowly and have fewer ions in the solution
B. Dissolve slowly and have an equal ion concentration in solution
C. Have a larger ion concentration in solution and dissolve more quickly
D. Have an identical ion concentration in solution but dissolve more quickly
8. By submerging it in a rhodium sulfate solution (Rh2(SO4)3(aq)), "white gold" is coated with rhodium to give it a white appearance. How long does it take to plate 3.0 10-5 g of rhodium onto a 3.0 g white gold broach when a 2.0 A current is available?
Note: The Faraday constant is 96,500 C/mol e-.
A. 0.56 s
B. 0.042 s
C. 0.0009 s
D. 0.0098 s
9. Why do halogens and alkaline earth metals frequently form ionic bonds?
A. The halogens and alkaline earth metals in the same row have different atomic radii.
B. Compared to halogens, alkaline earth metals have substantially higher electron affinities.
C. Halogens and alkaline earth metals both create entire octets by sharing electrons equally.
D. Compared to alkaline earth metals, halogens have substantially higher electron affinities.
10. What factors determine an element's atomic radius?
I. The quantity of valence electrons
II. The quantity of electron shells
III. The amount of neutrons in the nucleus
A. I only
B. II only
C. I and II only
D. I, II, and III
MCAT General Chemistry Passage 1
Multiple mechanisms working in the bloodstream contribute to blood pH equilibrium. They work together to keep the blood plasma pH around 7.4, as a drop below 6.8 or an increase above 7.8 may cause mortality.
The enzyme carbonic anhydrase, which catalyzes the conversion of blood CO2 to carbonic acid, is a part of this system. The buffer composed of carbonic acid and bicarbonate is created when carbonic acid ionizes.
Equation 1 below illustrates how these reactions are interdependent.
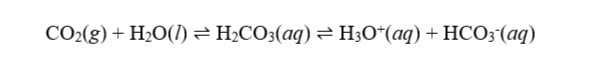
After oxygen is released in peripheral tissues, uncatalyzed blood CO2 and H+ are observed binding to hemoglobin. These processes are reversed when the blood reaches the lungs, when hemoglobin binds to oxygen and releases CO2 and H+ ions. The physiologic process of respiration involves the exchange of gases from the lungs with the blood and other bodily tissues.
In comparison to the carbonic acid-bicarbonate buffer, a second system, the phosphoric acid buffer, has less impact. The main reactant in this system is phosphoric acid (H3PO4), a triprotic acid that can ionize three protons. Below is an illustration of this three-step process:
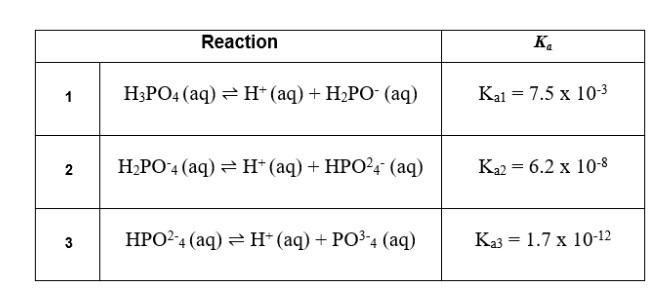
11. Which of the following would be TRUE if CO2 gas bubbled constantly in a beaker of water to create carbonic acid?
I. Adding carbonic anhydrase will raise the reaction's Keq.
II. Until Keq is reached, the concentration of carbonic acid will rise.
III. Adding bicarbonate will make the system's pH higher.
A. I only
B. II only
C. III only
D. II and III
12. Regarding human breathing, each of the following claims is accurate, EXCEPT:
A. CO2 builds up in the blood due to shallow, sluggish breathing.
B. Exercise prompts faster, deeper breathing, which raises the pH of blood plasma.
C. Excessive CO2 loss from hyperventilation can lead to the buildup of bicarbonate ions.
D. The blood [H+] level rises when a person's ability to breathe is hindered by illnesses like asthma or emphysema.
13. The trend Ka1 > Ka2 > Ka3 in the breakdown of phosphoric acid is mostly caused by:
A. A slower response rate following more ionizations
B. An increase in the anion's impact following subsequent ionizations
C. The H+ released in Reaction 1 has a smaller radius than the H+ released in Reactions 2 and 3
D. A balance shift in Reactions 2 and 3 in favor of the reactants as a result of the emission of H+ in Reaction 1
14. What is the pH of a solution made up of 10 mL of 0.15 M sodium acetate and 50 mL of 0.030 M acetic acid (Ka = 1.8 10-5)?
A. pH = 1.5
B. pH = 2.6
C. pH = 3.4
D. pH = 4.7
MCAT General Chemistry Passage 2
Atoms, which are the fundamental units of molecules, are made up of protons and neutrons around an electron-surrounded nucleus. The number of protons an atom has determines its identity. How many neutrons and electrons an atom has can have an impact on its stability and/or reactivity.
Quarks are little, fundamental particles that make up nucleons, which include protons and neutrons. Hadrons are composite particles made of quarks held together by a strong force. Protons and neutrons are held together in the nucleus by this powerful force, also known as the nuclear force, which outweighs any opposing forces that may be present. Two up quarks and one down quark unite to make baryons, which are hadrons made up of three quarks. One up quark and two down quarks combine to form uncharged nucleons. One quark and one antiquark makeup mesons, the other family of hadrons, which are unstable particles.
15. Twenty-nine hadrons, consisting of two down quarks and one up quark, and 28 hadrons, consisting of two up quarks and one down quark, are found in an atom. Which of the following best describes the nature of the atom?
A. Iron-57
B. Nickel-57
C. Nickel-58
D. Copper-57
16. Strontium has first ionization energies of 549.5 kJ/mol, second ionization energies of 1064.2 kJ/mol, and third ionization energies of 4138 kJ/mol, respectively. Why is ionization energy 3 so much higher than ionization energy 1 and ionization energy 2?
A. A fully occupied subshell is losing its third electron.
B. The third electron is more energetically intense than the first two.
C. In comparison to the first two electrons being eliminated, the third electron has a greater mass.
D. Compared to the previous two electrons, the third electron being removed has a lower nucleus attraction.
17. Similar charges repel one another whereas opposite charges attract one another. How is it possible for positively charged protons to dwell in the nucleus?
A. Hadrons do not interact with one another through forces.
B. An opposite force is created by the electron cloud in the area.
C. The nuclear force is more powerful than the forces that repel protons.
D. The nucleus' neutrons keep the protons from interacting with one another.
MCAT General Chemistry Passage 3
The origins of many caves can be traced to chemical principles. One type of cave, known as a solutional cave, is created when acidic water seeps through limestone (CaCO3, Ksp = 3.4 10-9 at 25°C). Caverns are created as a result of the dissolution of limestone during this process.
Beautiful rock formations are frequently found in solutional caves. They are the end result of equilibrium processes involving limestone, carbon dioxide, and water. Surface water entering the caverns comes into contact with dirt that has a higher PCO2 content than that of the atmosphere, which starts the process. This high CO2 concentration is the result of a process known as outgassing, which releases CO2 from the earth's mantle. The way that CO2 dissolves in water is seen in equation 1 below:
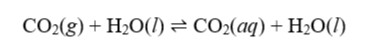
Equation 2 (shown below) describes how CO2(aq), once dissolved, contributes to the acidification of water.
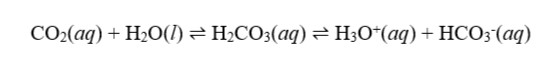
Finally, the acidic solution enters the cave through the roof and comes into contact with ambient air that has a lower PCO2 than the soil. This results in the gas release of the dissolved CO2(aq), which finally leads to the precipitation of CaCO3(s). This precipitate creates stalactites, which are downward spikes made by water pouring down from roofs toward the ground, and stalagmites, which are upward spikes created by water hitting the ground in the cave. The two can eventually meet to form a pillar in the cave over the course of thousands of years.
18. Groundwater resources close to a cave are unintentionally contaminated with (NH4)2CO3 by a nearby factory. What effects are most likely to have on the cave?
A. The creation of stalactites and stalagmites would probably not be impacted.
B. The production of stalactites and stalagmites would increase with increased groundwater acidity.
C. The creation of stalactites and stalagmites would be reduced by increased groundwater acidity.
D. Cannot be determined based on the provided data.
19. Stalagmite and stalactite growth is shown to decline when the temperature of the earth around a cave rises. Which explanation fits this the best?
A. CaCO3 melts when the temperature rises.
B. Stalactite and stalagmite formation is endothermic.
C. CaCO3 is less soluble in water as temperature rises.
D. As the temperature rises, the concentration of CO2(g) in the water increases.
20. Which of the following, when mixed with CaCO3(aq), will create a buffer?
A. CO2
B. C2H4O2
C. K2CO3
D. NaHCO3
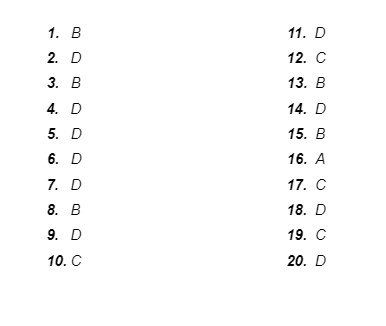
Answer Key:


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 




















