MCAT chemistry is one of the many science subjects you must learn to get ready to ace the MCAT test. It is among the most challenging subjects to learn for the MCAT.
It covers general chemistry, organic chemistry, and biochemistry as its three subtopics.
However, the day of the actual MCAT may cause unnecessary stress and anxiety; you may tend to forget what you have studied for. This is a strong possibility with many chemistry terms and equations to remember.
We do not want your effort in preparing and studying for the MCAT to go to waste. So, in this article, we share the best techniques and strategies as you review your answers for the MCAT chemistry section.What is MCAT Chemistry?
The three chemistry topics covered on the MCAT are general chemistry, organic chemistry, and biochemistry.
General chemistry refers to the study of matter, energy, and its interactions. Bases and acids, the periodic table, chemical reactions, atomic structure, and chemical bonding are some of the main topics in general chemistry.
The study of the structure, synthesis, reactions, and characteristics of molecules containing carbon is known as organic chemistry. The majority of organic molecules are composed of carbon and hydrogen, with the minor components being nitrogen, oxygen, halogens, silicon, phosphorus, and sulfur.
Lastly, cellular and molecular biological processes are the focus of biochemistry. The study of biochemistry was developed at the turn of the 20th century as a result of researchers combining chemistry, physiology, and biology to look into the chemistry of living systems.
Chemistry is the second-most studied subject on the MCAT after biology, making up 70% of the Chemical and Physical Foundations of Biological Systems (CHEM/PHYS).
In total, chemistry is covered by 41 questions (out of 59) in this section of the MCAT.
General Chemistry – 30%
First Semester Biochemistry – 25%
Introductory Physics – 25%
Organic Chemistry – 15%
Introductory Biology – 5%
In addition, 35% of chemistry is covered in the Biological and Biochemical Foundations of Living Systems (BIO-BIOCHEM). This means that, out of 59 questions, about 21 require your knowledge of chemistry.
First-semester Biochemistry – 25%
Introductory Biology – 65%
General Chemistry – 5%
Organic Chemistry – 5%
Summary Table of Chemistry Distribution in the MCAT
MCAT Section | Chemistry Subject | Percentage | Number of Questions (out of 59) |
|---|---|---|---|
Chemical and Physical Foundations of Biological Systems | General Chemistry | 30% | 18 |
First-Semester Biochemistry | 25% | 15 | |
Organic Chemistry | 15% | 9 | |
Biological and Biochemical Foundations of Living Systems | General Chemistry | 25% | 15 |
First-Semester Biochemistry | 5% | 3 | |
Organic Chemistry | 5% | 3 | |
Total Number of MCAT Chemistry Questions: 63 | |||
Chemistry Topics to Study for the MCAT
So that you may ace the MCAT and earn your target score, you must have a complete understanding of the ideas and chemistry knowledge areas assessed on it.
Here is a list of the different MCAT chemistry topics that you devote your attention to:
General Chemistry Topics to Study for the MCAT
Organic Chemistry Topics to Study for the MCAT
Biochemistry Topics to Study for the MCAT
How to Review for the MCAT Chemistry: 6 Useful and Proven Tips
Taking the MCAT could be nerve-wracking. No matter how you prepare and study for this life-changing exam – it is not a guarantee you will ace it.
However, even though the MCAT might seem challenging, it is much easier than you think. Understanding the various methods and techniques will help you answer the questions correctly.
We are here to help you on your MCAT journey and below are some of the best and proven tips and strategies you can apply as you review your answers in the MCAT chemistry.
Read the Questions and Understand What it is Asking
To avoid any procedural errors, thoroughly read the instructions before starting the exam. This is your chance to collect your thoughts and relax if you're feeling anxious.
To understand the inquiry, you must identify the keywords and the data that it requests. The exact subject being tested by the question must be specified, along with whether it is a direct or application question.
By choosing the topic, you can obtain a tip on the formulas and ideas to employ. If the query is technical, create a diagram to assist you in determining the answer.
Example:
There are multiple layers of gases with various properties and temperatures in the earth's atmosphere. The atmosphere is made up primarily of nitrogen, oxygen, argon, and carbon dioxide, with small amounts of water vapor. Its total mass is 5 1018 kg. 75% of this mass is located less than 11 km from the planet's surface. Although the Kármán Line, located about 100 km above sea level, is frequently accepted as the barrier, the line between outer space and the atmosphere is not clearly defined.
Gas | % of Dry Air |
|---|---|
Nitrogen | 78.1 |
Oxygen | 20.9 |
Argon | 0.93 |
Carbon dioxide | 0.038 |
The exosphere, thermosphere, mesosphere, stratosphere, and troposphere are the five principal layers of the atmosphere. The innermost layer, known as the troposphere, is located between the equator and the poles, where it rises to a height of about 20 km. The stratosphere is the following stratum. The stratosphere contains the ozone layer, which is thought of as a layer of its own due to its distinct composition. Although the actual quantities are fairly modest, the ozone layer contains about 90% of the oxygen in the atmosphere (2-8 ppm). This layer is crucial because it absorbs a significant amount of the ultraviolet (UV) light that the sun emits. The middle layer of the atmosphere, or mesosphere, has a temperature of roughly 100°C. The outermost layers are the thermosphere and exosphere, in that order.
With increasing altitude, the atmosphere's pressure, density, and temperature change. With rising altitude, both pressure and density are reduced. Air has a density of 1.2 kg/m3 at sea level and decreases by around 50% every 5.5 km.
The existence of greenhouse gases in the atmosphere, which accumulate and release heat infrared radiation, is what causes the greenhouse effect. Water vapor, methane, nitrous oxide, carbon dioxide, and ozone are examples of greenhouse gases. The earth would be substantially cooler if these gases were not present to keep it at its current temperature. However, it is believed that the rise in the average temperature over the twentieth century was caused by an increase in the amount of greenhouse gases in the atmosphere. A highly effective greenhouse gas is methane. With a 7-year half-life, it can be oxidized in the atmosphere to yield carbon dioxide and water.
The earth's atmosphere is true of everything below EXCEPT:
A. It is difficult to distinguish where the atmosphere ends and space begins.
B. Ozone gas makes up the majority of the ozone layer.
C. Within 11 kilometers of the earth's surface, there are more than 3.75 1018 kg of atmospheric gases.
D. The presence of water vapor makes the atmosphere warmer.
Explanation:
Take note that the question has the word ‘EXCEPT’, meaning you are required to choose the option that does NOT belong among the given options.
The passage states that the limit between outer space and the atmosphere is not definite. Hence option A is a truthful statement.
The passage also claims that the atmosphere has a mass of 5 1018 kg, of which 3.75 1018 kg, or 75%, are located 11 kilometers above the earth's surface. Option C is also true because of this.
Since water vapor was mentioned as a greenhouse gas in the previous sentence, option D is accurate. The passage claims that ozone concentrations range from 2 to 8 ppm, despite the fact that the bulk of ozone is found in the ozone layer. Concentrations would need to be greater than 500,000 ppm to be the main component.
Therefore, option B - Ozone gas makes up the majority of the ozone layer, is a false statement, making it the right response.
Make Smart Guesses
When there are only two or three contested choices left after analyzing the answers to MCQ (multiple-choice) questions, you can find yourself in a challenging scenario. Use your abilities to predict to save time when this happens.
However, since speculation depends on chance, make every effort to reduce it as little as possible. Remember that your initial response was likely true as you proceed and stick with it.
Example:
Refer to the previous passage for the text.
How long would it take for atmospheric oxidation to reduce a 1 L sample of methane to 1% of its initial volume?
A. 38 years
B. 42 years
C. 47 years
D. 50 years
Explanation:
Methane has a 7-year half-life, according to the text. Six half-lives would leave 1.5 percent of the methane remaining and seven half-lives would leave 0.8 percent of the methane remaining after starting with 100 percent and dividing by two for each half-life.
Therefore, it would take 42 to 49 years, and the correct answer is C - 42 years.When Solving an Equation, Adhere to the Step-by-Step Process
Simply follow the steps for solving word problems if you are having issues with something. This implies that you must first determine what the inquiry is asking.
Next, determine the equation that must be used in conjunction with the information provided for you to arrive at the right solution.
This is a really well-organized and efficient strategy to use for all calculation-intensive MCAT problems, not just chemistry-related ones.
Remember that every detail must be accurate for this method to work properly; otherwise, you will receive an erroneous result.
Example:
A steel container contains an unknown number of moles of argon. Two moles of nitrogen are injected by a chemist into the container. While neither the temperature nor the volume change, the pressure does go up by 10%. Initially, the container contained:
A. 16 moles of Ar.
B. 18 moles of Ar.
C. 20 moles of Ar.
D. 22 moles of Ar.
Explanation:
To correctly respond to this query, you first need to identify what the question is asking for, the data given, and the equation needed.
The question asks the number of moles contained at the beginning. You are given the number of moles of nitrogen and the pressure.
The pressure of an ideal gas, under constant V and T, represents the number of particles (regardless of their identity). PV = nRT to P n is a simplification of the ideal gas law.
As a result, if two moles of N2 are introduced to the chamber and P increases by 10%, the additional moles must equal 10% of the initial amount of Ar moles. A percentage of 20 moles is 10%, or two moles.
Therefore, the correct answer is C - 20 moles of Argon.
Examine and Analyze Every Response
This entails looking at each response's criteria to rule out potential outcomes.
One can filter the options and eventually come up with just one answer by removing any irrelevant responses.
While doing this, go back to the initial question frequently to make sure you're just eliminating responses that aren't relevant.
Example:
Which among the following statements best sums up how the Heisenberg uncertainty principle prevents us from measuring position and momentum precisely and simultaneously?
A. Limitations of current scientific tools' accuracy
B. Inaccurate measurements of the meter and kilogram
C. Measurement of one variable increases the error in the other
D. Difference in mass between a nucleus' mass and that of its constituent particles
Explanation:
The Heisenberg uncertainty principle has restrictions because measurement itself has restrictions. For example, if a particle is moving, it has momentum, but attempting to measure that momentum inherently introduces uncertainty in the position.
Even if we had an exact definition of the meter, as in option A, or ideal measuring tools, as in option B, we wouldn't be able to precisely and simultaneously measure position and momentum.
Therefore, the correct answer is C - Measurement of one variable increases the error in the other.
Focus on the Diagrams
Nearly all MCAT organic chemistry questions may be resolved using only reaction diagrams.
Try skipping the paragraph and go straight into the questions. You might be shocked to discover that you can save a ton of time and still answer the questions correctly.
As you focus on the diagrams, ensure that you understand what skills are being tested. Remember that the MCAT focuses on applying what you have learned during your preparation period.
Example:
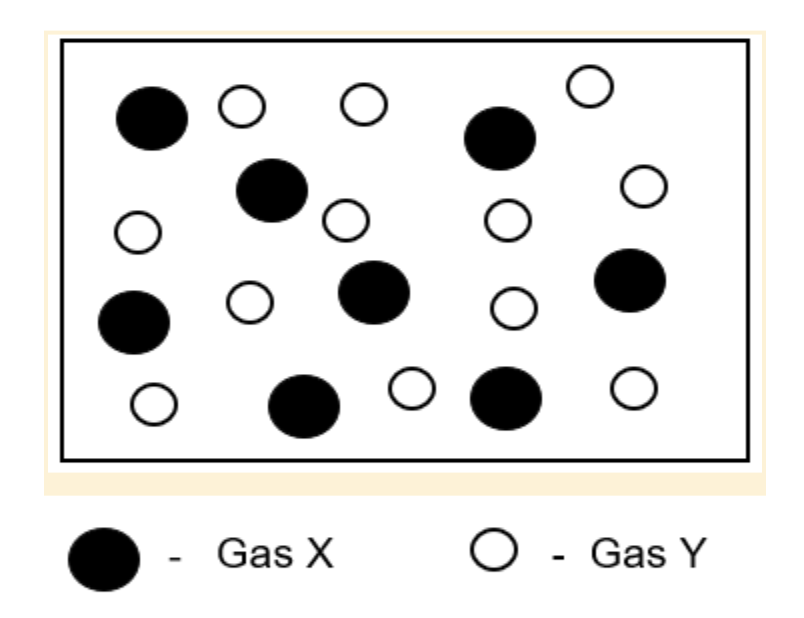
The relative diameters and mole fractions of two monatomic gases in a sealed container are shown in the image below.
Which statement regarding the gas combination is accurate after a tiny hole is drilled in the container and the gases are given time to fully effuse?
A. Each gas will continue to have the same partial pressures.
B. Gases X and Y will never have partial pressures that are equal to each other.
C. Gas Y's partial pressure will drop more quickly than Gas X's partial pressure.
D. As the mole fraction of Gas Y increases, so will the partial pressure of Gas Y.
Explanation:
Take note that you are being asked of an effect after an action and therefore you have to analyze and explore possible options.
The total pressure of the container will drop after a hole is made in it, and the gases start to leak out. As a result, each partial pressure of the gases will likewise drop. Choose neither A nor D.
According to Graham's law, a gas molecule will effuse through a small hole at a faster pace the lighter it is. Gas Y will effuse more quickly than Gas X, which will cause its partial pressure to drop more quickly.
Once the gases have fully effused, the figure shows that Y's mole fraction is somewhat larger than X's. Since the container will eventually be empty at some point, option B will therefore be ruled out at some point when the partial pressures of both gases are equal.
Therefore, the correct answer is C - Gas Y's partial pressure will drop more quickly than Gas X's partial pressure.
Apply the Elimination Technique
We always advise locating the passage section that applies to the particular issue after reading the question.
Reread that section to see if you require any additional details to respond to the question.
When you are certain an option is incorrect, start by crossing it out. After that, you ought to be able to rule out the alternatives and decide on the best option.
Example:
Which of the following accurately describes Zn2electron +'s configuration?
A. 1s22s22p63s23p64s03d10
B. 1s22s22p63s23p64s23d8
C. 1s22s22p63s23p64s23d10
D. 1s22s22p63s23p64s03d8
Explanation:
As you remove electrons from an element to create a cation, keep in mind that they will first leave the subshell with the largest n value.
Given that Zn0 has 30 electrons, its electron configuration would be 1s22s22p63s23p64s23d10.
The 4s subshell is emptied first since it has the highest main quantum number, resulting in the formation of 1s22s22p63s23p64s03d10.
Option C gives the configuration of the uncharged zinc atom, option D displays the configuration that would be present if four electrons were removed, and option B suggests that electrons are taken out of the d orbital.
Therefore, the correct answer is A - 1s22s22p63s23p64s03d10.How Much Time Should You Give Yourself to Study for the MCAT Chemistry?
The amount of time you dedicate to studying could affect your MCAT score.
Therefore, it is something that you should properly plan for before you start studying. Those students who perform well usually devote 3 to 6 months and six hours per day to MCAT preparation.
However, depending on where you begin, you might only require half as much time (or twice as long).
The majority of the MCAT is made up of chemistry and physics. A total of 63 questions on the MCAT call for knowledge of chemistry. You must put a lot of effort into your MCAT chemistry studies. These 63 questions may determine your route to medical school.
As was previously indicated, 6 hours should be dedicated to MCAT preparation over 3–6 months.
An optimal MCAT study schedule would suggest that you focus on three subjects each day. It means that each subject—in this case, MCAT chemistry—should receive 2 hours of time.
Depending on your obligations at work or school, you might need to make adjustments from time to time.
However, don't forget to set aside a day each week for rest or recreation. You don't want to be worn out and overworked when you take the MCAT.
Additional FAQs – Review for the Chemistry Section in the MCAT
How Can I Improve My Chemistry MCAT Score?
There is a large range of topics covered by chemistry (general chemistry, organic chemistry, and biochemistry).
Due to time limits, you cannot cover them all. Concentrate on important subjects only.
Additionally, you can make use of flashcards and mnemonic devices. You can use them to help you remember key concepts and formulas for the MCAT.
How Much Chemistry Do You Need for the MCAT?
It is covered in both the MCAT Bio/Biochem and Chem/Physics sections.
Study general chemistry, organic chemistry, and biochemistry as you prepare for the MCAT.
To determine the subjects you should pay closer attention to, check out the list of subjects we have in this article.


 To help you increase your MCAT score to the competitive mark quickly, we have collaboratively created these
To help you increase your MCAT score to the competitive mark quickly, we have collaboratively created these 




















