I. What is Protein Structure?
Can we talk about a throwback?! It’s no doubt if you were (or are) a biology or biochemistry major, this is a topic, along with amino acids, you may know like the back of your hand! But whether you have a biology/biochemistry degree or are a non-traditional English major, this is without a doubt a must-master topic for the MCAT.
Aside from being a must know topic that will undoubtedly come up on the MCAT, these serve as the basis for all basic physiological functions in the human body, while also having many ties with our topics of the MCAT outside of biology!
This is a definitely high yield topic with protein structure that covers all aspects of what gives a protein its shape and oftentimes its function, including the sequence of amino acids all the way to their 3D folding. Let’s dive in & tackle this topic together!
II. Basics of Protein Structure
Let’s first start off with understanding the underlying interactions that govern protein folding There are 4 main levels of protein structure, each with their own underlying interactions: primary (1˚), secondary (2˚), tertiary (3˚) and, quaternary (4˚) structure.
A. Primary (1˚) Structure
In its simplest form, a protein’s structure refers to the linear sequence of amino acids which are connected by peptide (amide) bonds. Think of them as like a line of amino acids!
The 1˚structure actually contains all the information needed for 3D protein folding as we’ll see when talking about 3˚structure later!
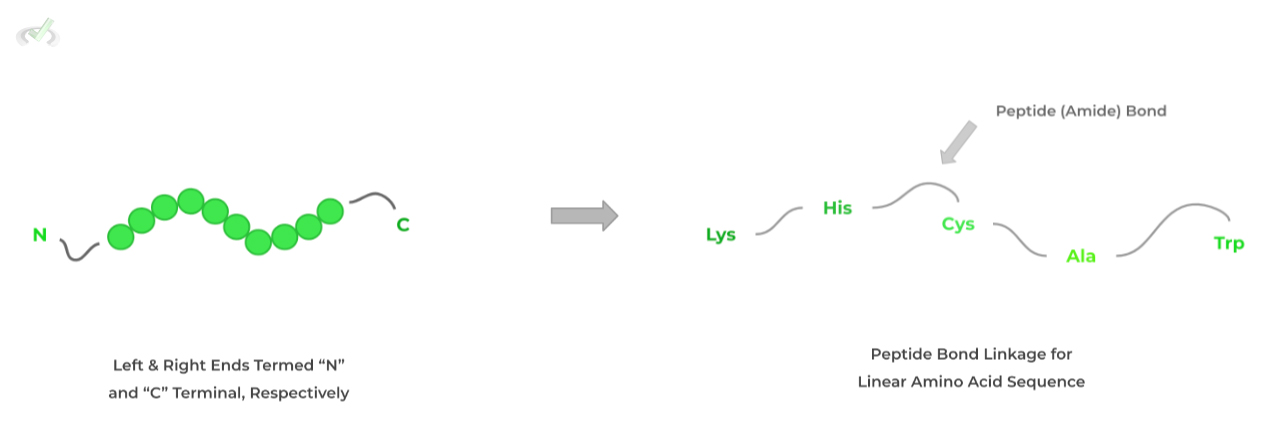
When reading the sequence, the left end terminates with an amine group termed the N-terminal while the right terminates with a carboxyl group termed the C-terminal.
These are important in proteolytic reactions where proteases cleave the peptides at certain terminals!B. Secondary (2˚) Structure
A good way to think of 2˚structure is a precursor folding before the fully, 3D protein folding in 3˚structure, which is driven by the interactions between the amino acids’ amide groups.
Specifically, the amide groups form hydrogen bonds where one of the amino’s hydrogen forms a hydrogen bond dipole with the carbonyl oxygen as shown below.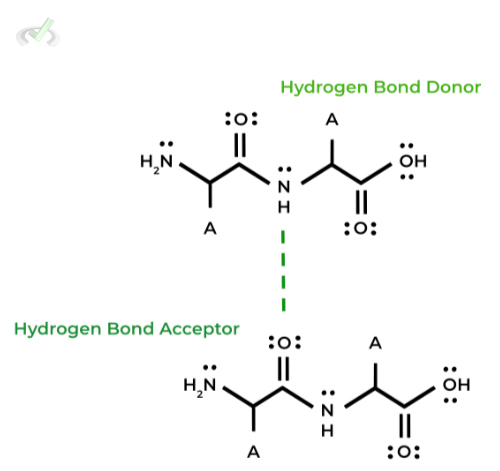
Furthermore, there are 2 types of 2˚structures that’ll be most likely tested on the MCAT: 1) alpha (𝜶) helix and 2) beta (𝜷) pleated sheets. Let’s discuss them more below!
I. Alpha (𝜶) Helix
Similar to the DNA helix, a polypeptide chain can form a similar helix structure where the hydrogen bond interaction within the helix with the side chains protruding outwards.
This is a fairly common 2˚structure motif found in proteins such as in G coupled protein receptors where 7 alpha helix structures span the membrane!II. Beta (𝜷) Pleated Sheets
In this other common 2˚structure motif, the polypeptide chain takes a more planar form like a sheet of paper where the hydrogen bonds are formed side by side and the side chains are protruding above and below the sheet.
In addition, the protruding side chain groups cause the sheet to actually have a “ripple” shape as opposed to strictly planar form.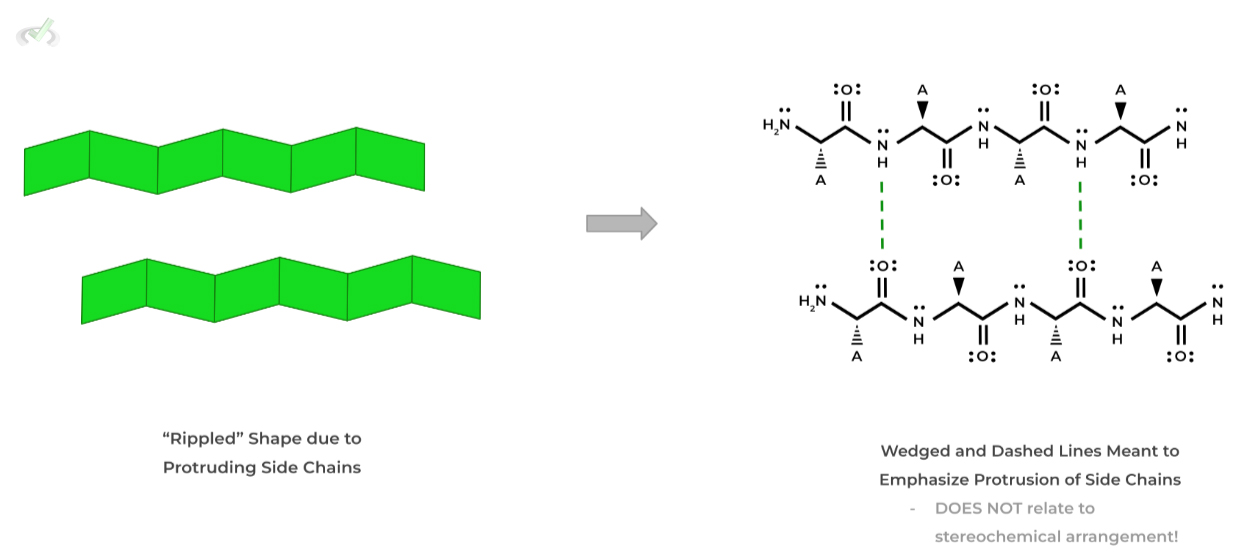
Beta-sheets can also be classified depending on the terminal end orientation of the strands!
If the strands have the SAME terminal ends, they form a parallel orientation whereas those with DIFFERENT ends form an antiparallel orientation.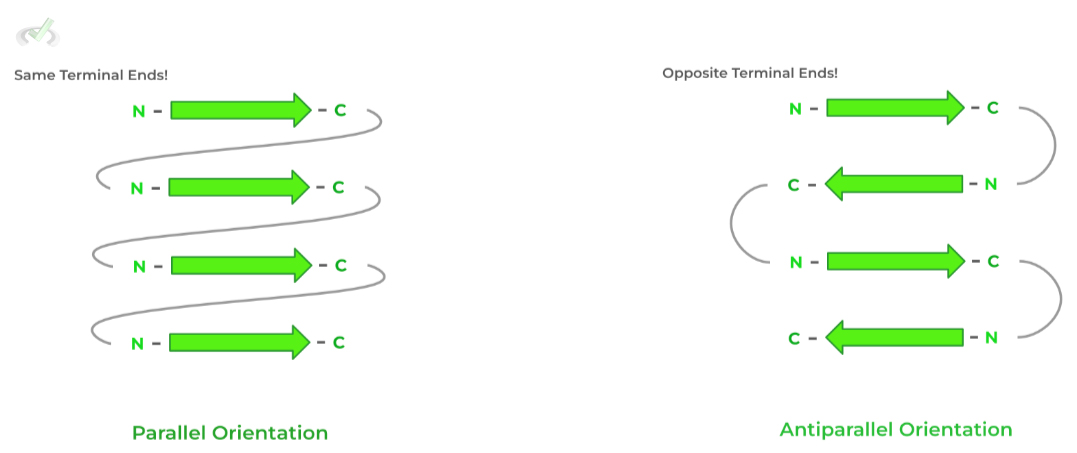
C. Tertiary (3˚) Structure
This level of protein structure most highly contributes to the 3D protein folding, where the primary interactions occur between amino acid side chains.
There are many interactions that govern 3˚ structures including dipole-dipole, hydrogen bonds, salt bridges, van der Waals, etc.I. Conformational Stability in Hydrophobic Effect
A key driver of protein conformational stability is the hydrophobic effect, which states that water molecules want to maximize their disorder by minimizing their interactions with hydrophobic molecules.
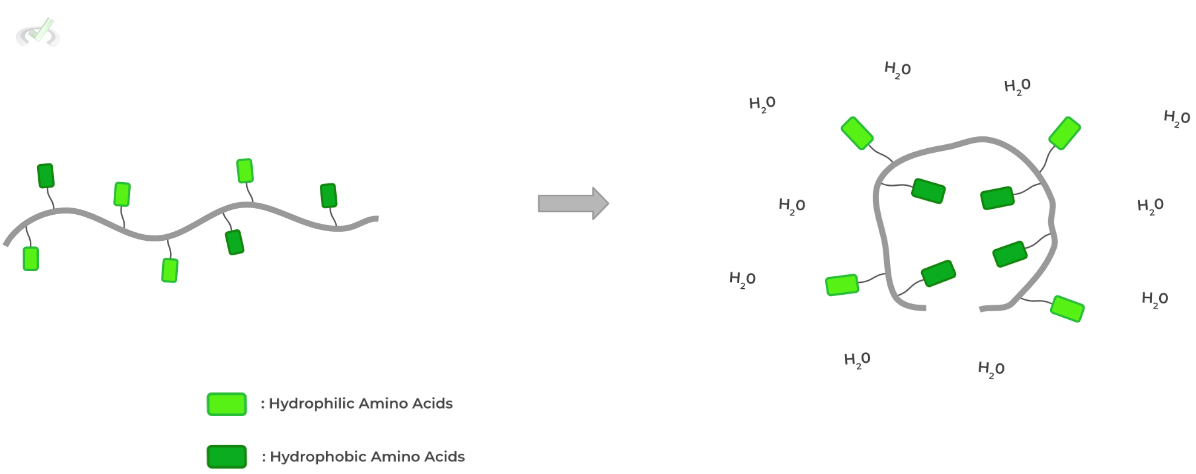
As such, cytosolic proteins fold in a way where hydrophobic amino acids are located in the core while hydrophilic ones are found on the protein surface!
Here’s some food for thought! How will this be different for a membrane protein where its protein surface interacts with the hydrophobic membrane core?D. Quaternary (4˚) Structure
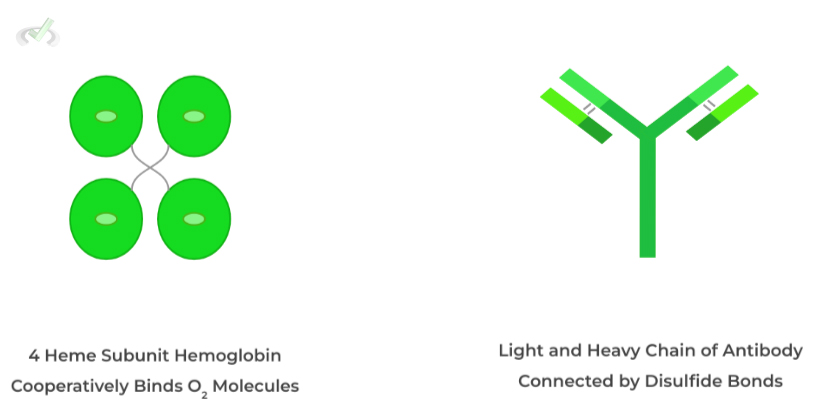
This structure actually involves the interaction of multiple polypeptide, protein subunits to create a bigger functional protein!
Much of the interactions in 4˚structure are similar to those found in 2˚and 3˚ structure such as hydrogen bonds, salt bridges, and disulfide bonds with the last one being fairly important! Let’s give a few examples!
Hemoglobin contains 4 protein subunits each with an heme group and center iron atom while antibodies have a light and heavy chain connected by disulfide bonds!
E. Environmental Effects on Conformational Stability
External conditions due to the environment can also affect the conformational stability of proteins, most notably temperature, pH conditions, and salinity!
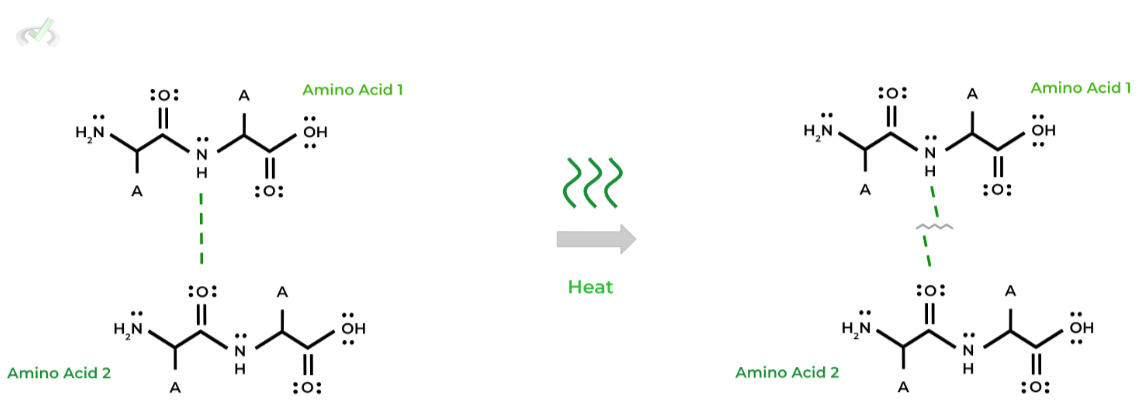
I. Temperature
Generally speaking, an increase in temperature of the protein’s environment DENATURES the protein due to the breaking of hydrogen bonds within the proteins structure!
II. pH Conditions
Similarly, changes in the pH of the protein’s environment also has the capacity to denature the protein as well in various interactions, such as hydrogen bonds and salt bridge interactions!
This is due to changes in the protonation states of functional groups which can alter the above mentioned interactions. Look at the example below!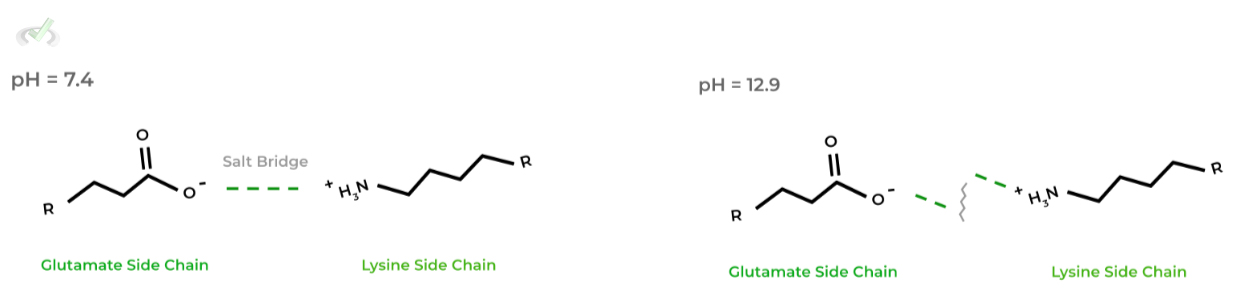
As shown, In physiological conditions, the charges glutamate and lysine are negative and positive, respectively, allowing them to form an electrostatic salt bridge interaction.
However, more basic conditions (pH = 12. 9), lysine becomes deprotonated and loses its positive charge disrupting the salt bridge!III. Salinity
Finally, the salinity of the protein’s external environment also has the capacity to denature the protein, most notably salt bridge interactions!
The dissociated ions can also form electrostatic interactions with the charged functional groups and side chains, disrupting the already formed salt bridges.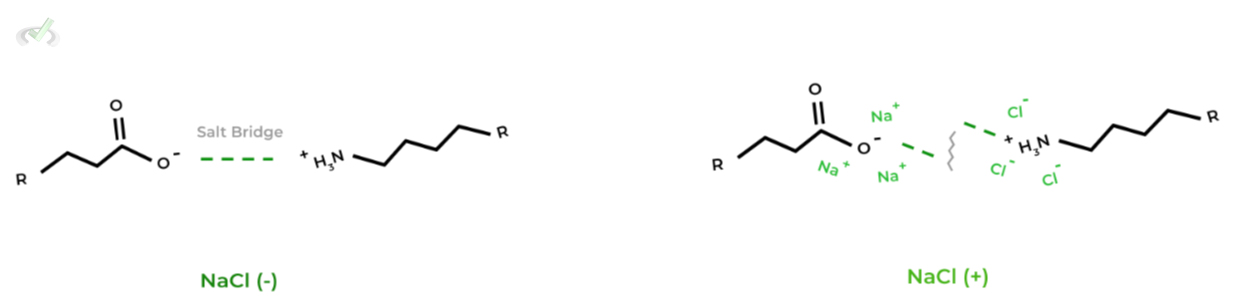
III. Bridge/Overlap
As a supplement to understanding protein folding, it may also be helpful to get a quick review of some of the many interactions that make up 2˚, 3˚, and 4˚structure. We’ll skip over the peptide bonds form in 1˚structure as that has its own article!
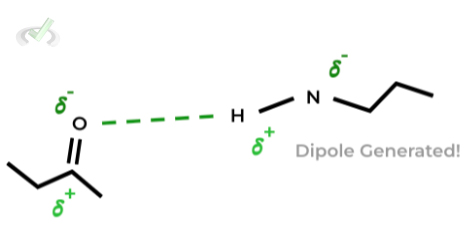
I. Hydrogen Bonds
These bonds are a specialized type of dipole-dipole where a hydrogen atom bound to a F, O, or N atom forms a hydrogen bond with another F, O, or N atom!
Remember this is due to the uneven distribution of electrons which favors the F, O, and N atoms due to their electron affinity,
This leads to the F, O, and N atoms harboring a negative dipole while the hydrogen atom has a positive dipole.
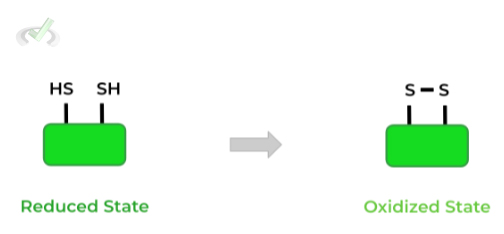
II. Disulfide Bonds
These bonds are a special type of covalent bond which results from the oxidation of the thiol/sulfhydryl groups of 2 cysteine residues as shown below!
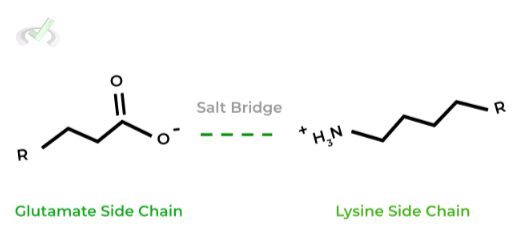
III. Salt Bridges
As their name implies, these amino acid interactions are very similar to ionic salts as their also form electrostatic interactions due to their opposite charges! It’s best to know and memorize the acidic and basic amino acids!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Primary (1˚) Structure
B. Secondary (2˚) Structure
I. Alpha (
C. Tertiary (3˚) Structure
D. Quaternary (4˚) Structure
E. Environmental Effects on Conformational Stability
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
𝛽-mercaptoethanol (BME) is a common reducing agent utilized within reducing SDS-PAGE, which works to break disulfide bonds. Which of the following protein structures should not be affected by 𝛽-mercaptoethanol (BME)?
I. Primary (1˚)
II. Secondary (2˚)
III. Tertiary (3˚)
IV. Quaternary (4˚)
A. I only
B. IV only
C. I & II only
D. II & III only
Ans. C.
For this question, you must know the main interactions that govern the different levels of protein structures. Below shows the summarized interactions for the protein structures:
Primary (1˚): Peptide (Amide) Bonds
Secondary (2˚): Hydrogen Bonds between Peptide Bonds
Tertiary (3˚): Multiple
-Disulfide Bonds
-Salt Bridges
-Dipole
-Hydrophobic
-Hydrogen Bonds
Quaternary (4˚): Multiple (same as tertiary)
Because BME works to break disulfide bonds, this will ONLY affect the tertiary (3˚) & quaternary (4˚) structure, as these are the only levels of protein structure that contain disulfide bonds. Thus, the only protein structures that WON’T be affected are the primary (1˚) & secondary (2˚) structure.
Sample Practice Question 2
In order for steroid derived hormones to act on their target organs, they will have to utilize a transport protein in order to be transported in the bloodstream. This is because they need a soluble protein in the bloodstream because the steroid hormone is nonpolar and cannot dissolve easily in the bloodstream as peptide hormones. Which of the following pairs of amino acids would you expect to be on the transport protein’s surface?
A. E & S
B. G & L
C. P & F
D. I & V
Ans. A.
What’s important to know in this question is that the bloodstream is a very hydrophilic environment, as a big portion of blood is water, around 90% of blood plasma (plasma itself makes about 55% of blood). Thus, the transport protein is similar to a cytosolic protein.
Thus, we should expect that hydrophilic amino acids (polar, positively charged, negatively charged) should be on the surface while hydrophobic amino acids should be within the core.
This makes sense because the amino acids in the core will partake in hydrophobic interactions with the nonpolar steroid hormone.
VI. Additional Links
Here are some additional links to related articles & additional resources that will hopefully be helpful to you all!
“Protein & Amino Acids” Quicksheets.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























