I. What are the Thermodynamics of Biological Systems?
Because biological systems are constantly participating in ongoing biochemical reactions, the role of thermodynamics in biological systems is highly important and is a good way to put ideas in general chemistry in a biological context.
Recall that thermodynamics is the branch of general chemistry that deals with the transfer of energy. Hopefully after this general definition, the application of thermodynamics in a biological, cellular context is a bit more clearer now!
To be honest when going through and reading this article, it may seem like a quick summation of thermodynamics if you’ve by chance have already started prep in general chemistry. However, we’ll make sure to also include different biochemical reactions as examples to better see the overlap!
II. Energy Changes in Biochemical Reactions
It’s impossible to discuss and review the thermodynamics in biological systems without discussing Gibbs free energy! Let’s see how this concept applies to biochemical reactions!
A. Gibbs Free Energy
Every biochemical reaction has an associated change in Gibbs free energy which can tell us the spontaneity of the reaction!
Recall that if the value of 𝜟G is negative, the reaction is spontaneous and is termed exergonic as it release energy into the surrounding environment. Let’s look at the common example of ATP hydrolysis!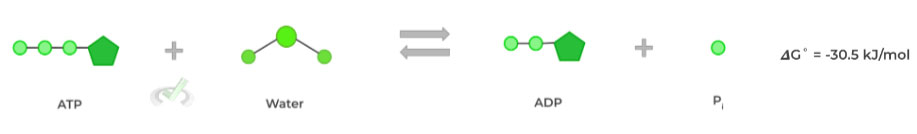
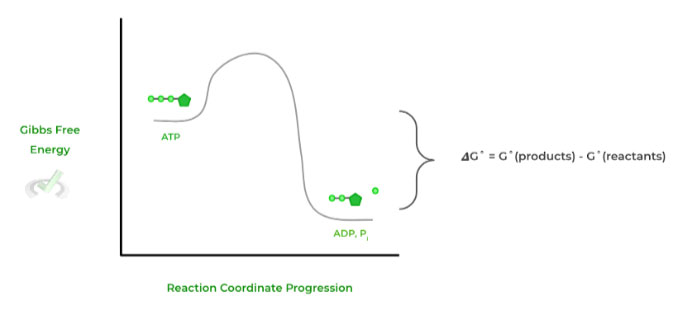
Here, the negative 𝜟G˚ value indicates that the reaction is spontaneous and exergonic, meaning that no external energy input was required for the reaction to proceed.
This can be best depicted in an a free energy diagram, where the value of 𝜟G˚ is simply the difference between the free energy states of the products and reactants.
Conversely, if the value of 𝜟G is positive, the reaction is nonspontaneous and is termed endergonic as it external energy is required to power the reaction. Let’s look at the example of sucrose synthesis from glucose and fructose!

With a positive 𝜟G˚ value, the reaction is nonspontaneous and endergonic, where extra external energy source is needed to power the reaction.
As shown in the reaction diagram, the products are at a higher energy state than the reactants due to the external energy input.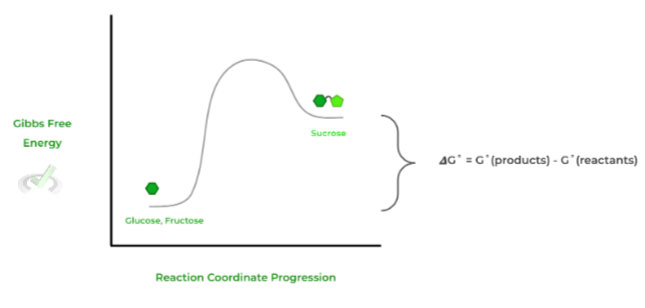
III. Bridge/Overlap
Why are some reactions spontaneous while others are nonspontaneous? While there are a variety of reasons, one important factor is the molecule stability between the reactants and products. Let’s take a look at how this plays out!
I. Effect of Molecular Stability on Reaction Spontaneity
One general rule of thumb for molecule stability is that high energy molecules are generally less stable while low energy molecules are more stable.
Combine this with another general concept that molecules want to increase their stability and therefore head to a lower energy state. Let’s look at ATP hydrolysis as an example by first looking at the structure of ATP.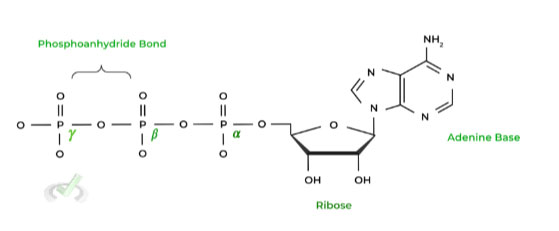
Recall that ATP is a high energy molecule due to the electrostatic repulsion between the adjacent phosphate groups, with these repulsions making the molecule unstable.
Now it makes sense why ATP hydrolysis is a spontaneous reaction: ATP wants to resolve this instability by losing one (or more) of its phosphate groups.
By losing the phosphate group(s), the electrostatic repulsion is minimized making the molecule more stable and lowering its energy state!IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Gibbs Free Energy
The value of 𝜟G can confer the spontaneity of a biochemical reaction and whether is released or inputted into the system!
A negative value for 𝜟G indicates that the reaction is spontaneous and exergonic, meaning that energy is released during the reaction, such as in ATP hydrolysis.
A positive value for 𝜟G indicates that the reaction is nonspontaneous and endergonic, meaning that energy is inputted in order to power the reaction, such as in the synthesis of sucrose from glucose and fructose.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
What should the reaction diagram look like for an exergonic reaction and why?
A. The products are at a lower energy state than the reactant because energy is released
B. The products are at a lower energy state than the reactants because energy is gained
C. The reactants are higher in energy than the products because energy is gained
D. The reactants are lower in energy than the products because energy is released
Ans. A
Be careful with the wording for the phrases above! In a spontaneous, exergonic reaction, energy is released resulting in the products being at a lower energy state than the reactants!
Sample Practice Question 2
Researchers induce numerous mutations to a protein’s transmembrane domain where instead of its normal polypeptide sequence of L-A-V-Y, the mutated sequence results in L-E-S-H. When inserted into the membrane, what can be said about the energy state of the mutated protein compared to the normal, wildtype protein and why?
A. Lower Energy State; because the molecule is less stable
B. Lower Energy State; because the molecule is more stable
C. Higher Energy State; because the molecule is less stable
D. Higher Energy State; because the molecule is more stable
Ans. C
Recall that generally higher energy molecules are unstable and tend to want to return to a lower state to minimize their stability. The mutated protein sequence contains polar and charged amino acids.
When placed into the membrane, this would form unfavorable interactions increasing both the instability and energy state of the protein!



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























