I. What is Cellular Membrane Transport?
In this article, now we really get into the function of the cellular membrane: it’s highly important role in selectively transporting the necessary nutrients, compounds, and substances that the cell needs in order to survive and thrive.
We already briefly introduced the relatively simple transport (a little bit of foreshadowing) of nonpolar molecules through the membrane in a previous article: now we’ll focus on the other types of transports that are involved for other molecules.
Without a doubt, this is one of the sections that has the most potential for overlap! With cells engaging in the constant back and forth transfer of particles and molecules, it’s no wonder that the concepts in this article are found in our daily biology!
II. Solute Transport Across Membrane
Membrane transport of particles can be categorized in 2 ways: 1) passive transport and 2) active transport, with the main difference being the requirement of energy in the transport. Let’s take a more in depth review of each below!
A. Passive Transport (High to Low)
This type of transport DOES NOT require the input of energy and thus occurs spontaneously. Passive transport involves the movement of a substance FLOWING DOWN its concentration gradient.
In other words, the substance will move from an area of high concentration to one of low concentration. This movement reduces the energy of the system which is why no energy input is needed.
Passive transport can further be subdivided into simple and facilitated diffusion, where the former DOES NOT require a protein & the latter DOES require a protein to facilitate transport!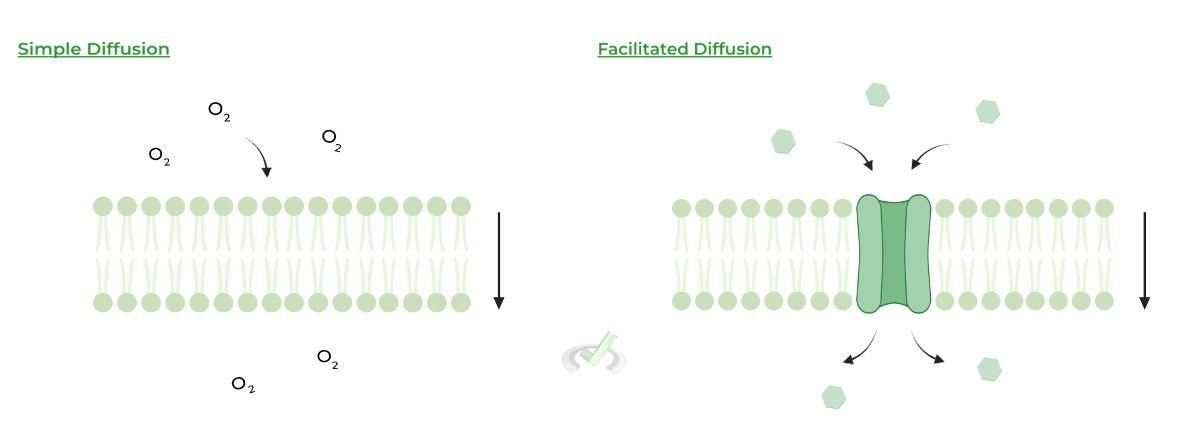
Notice how in both examples, there is a higher concentration of the molecules on the outside compared to the inside; as such, the molecules flow down their concentration gradients when moving inside.
Simple diffusion typically involves small, nonpolar molecules due to their size and favorable hydrophobic interaction. Bigger, polar molecules will usually partake in facilitated diffusion for the opposite reasoning.I. Osmosis
This type of transport specifically involves the movement of water molecules to areas of higher solute concentration (i.e. higher osmotic pressure/gradient)!
This can actually be described as both simple and facilitated diffusion because this movement requires NO ENERGY and can either diffuse through the cell membrane or through an aquaporin channel protein.
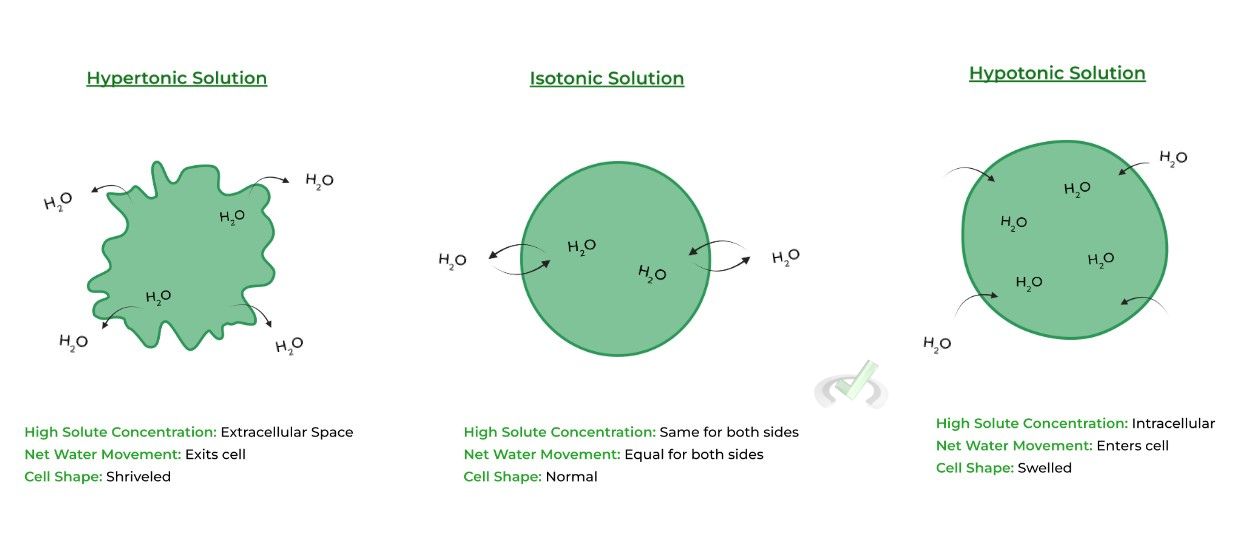
The type of environment a cell is placed in can have specific terms depending on the direction of net water movement in osmosis as listed below!
Though water is polar, a small portion of water molecules can simply diffuse through the membrane because of its small structure. Most water molecules will participate in facilitated diffusion utilizing aquaporins due to its polar nature.
B. Active Transport (Low to High)
In active transport, energy IS REQUIRED to transport particles across the membrane. This is because particles are moving UP AGAINST their concentration gradient.
Likewise, substances move from an area of low concentration to one of high concentration; this increases the energy in the system, making it nonspontaneous
Similar to passive transport, active transport can also be subdivided into 2 categories based on the source of their energy: primary (1˚) and secondary (2˚) active transport.I. Primary (1˚) Active Transport
Utilizes a DIRECT source of energy, most often than not coming from the hydrolysis of ATP, to power the movement of the substance! A great example would be the Na+/K+ ATPase pump!
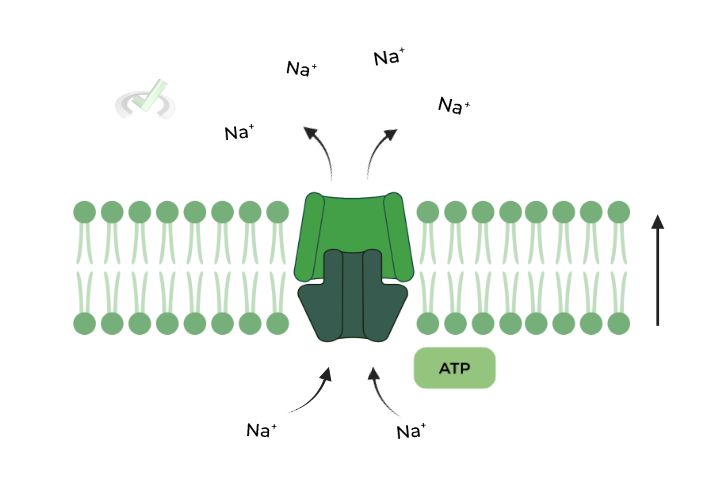
Most of the proteins involved in primary active transport will have dual enzyme and transport function to simultaneously hydrolyze ATP for energy and transport the substances.
II. Secondary (2˚) Active Transport – Coupled Transport
Utilizes an INDIRECT source of energy, which comes from the flowing of another substance DOWN its concentration gradient. It’s much easier to explain with a visual example!
In the below case, glucose is against its concentration while Na+ flows with and down its concentration gradient.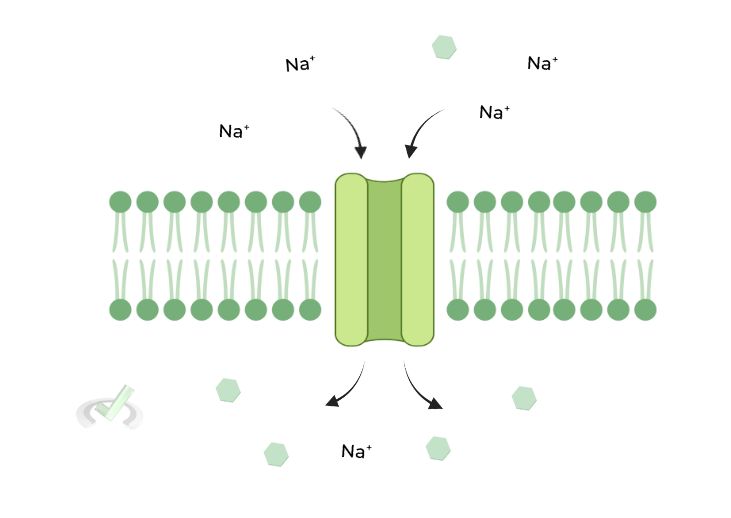
The energy released through Na+ spontaneously flowing down its concentration is used to power the “uphill” transport of glucose against its concentration gradient.
Transport proteins can sometimes transport 2 particles at once! A symporter transport 2 substances in the SAME direction while an antiporter transport them going OPPOSITE directions! The 1˚ and 2˚ active transport examples shown above display an antiporter & symporter protein, respectively!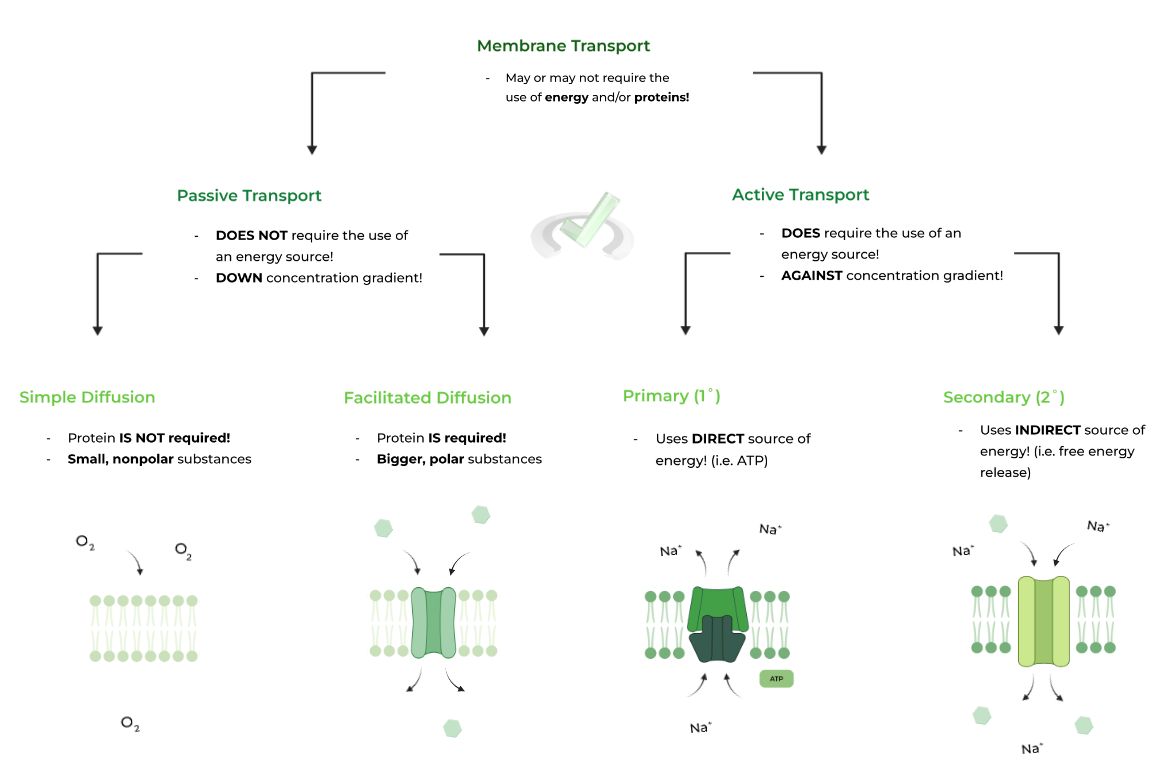
C. Vesicular Transport – Exocytosis, Endocytosis, and Pinocytosis
While there are many more caveats and intricacies, we’ll mainly focus on what happens on the cell surface! Have a look at all the terms defined and visualized below!
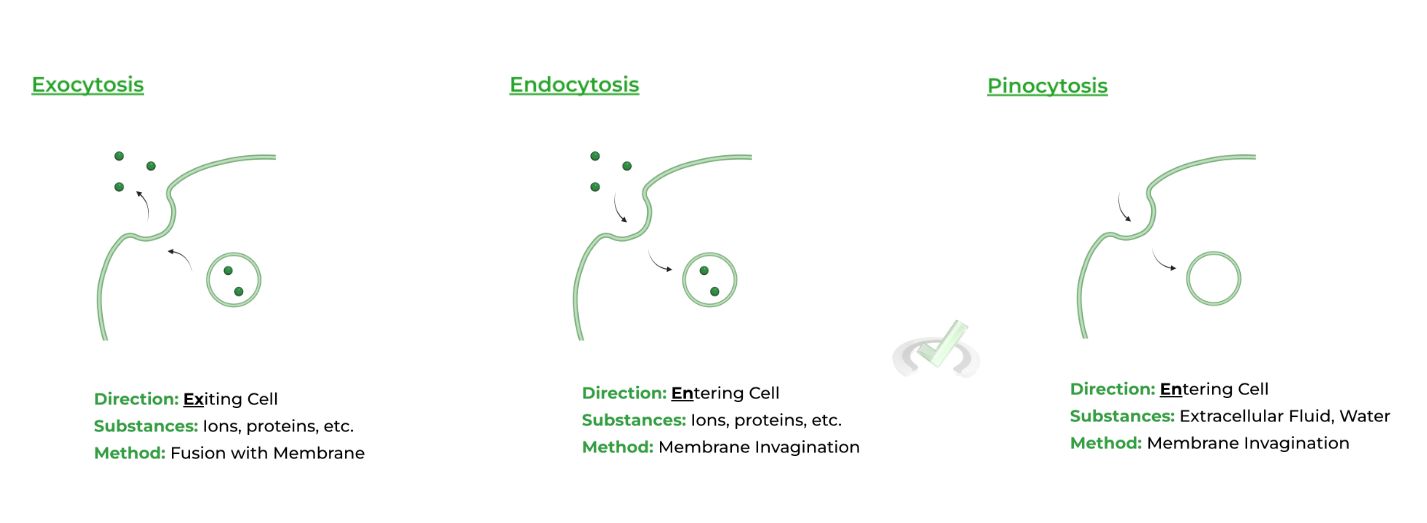
In endocytosis and pinocytosis, think of the cell membrane invaginations as a “pinching” of the cell membrane, like pinching of a piece of dough! As such, think of exocytosis as “fusing” with the membrane, like merging a piece of dough back with the larger lump!
These vesicles are crucial when discussing the secretory pathway as well as receptor mediated entry for viruses, covered in more depth in other articles!
III. Bridge/Overlap
We noted that 2˚ active transport requires two particles: one flowing down its concentration gradient (high to low) and one going against (low to high). Let’s use a little thermodynamics and visuals to get a better idea of why this works!
A. Exergonic and Endergonic Movement
These terms describe processes , most often a reaction, and its change in energy state within the system. Exergonic refers to the release of energy from a system while endergonic refers to the input of energy.
Thus, an exergonic process will have a low energy state when finished and an endergonic one will have a high energy state. Let’s apply this now!
The passive, facilitated transport (high to low) of Na+, is EXERGONIC and releases energy! This released free energy then powers the ENDERGONIC active transport (low to high) of glucose!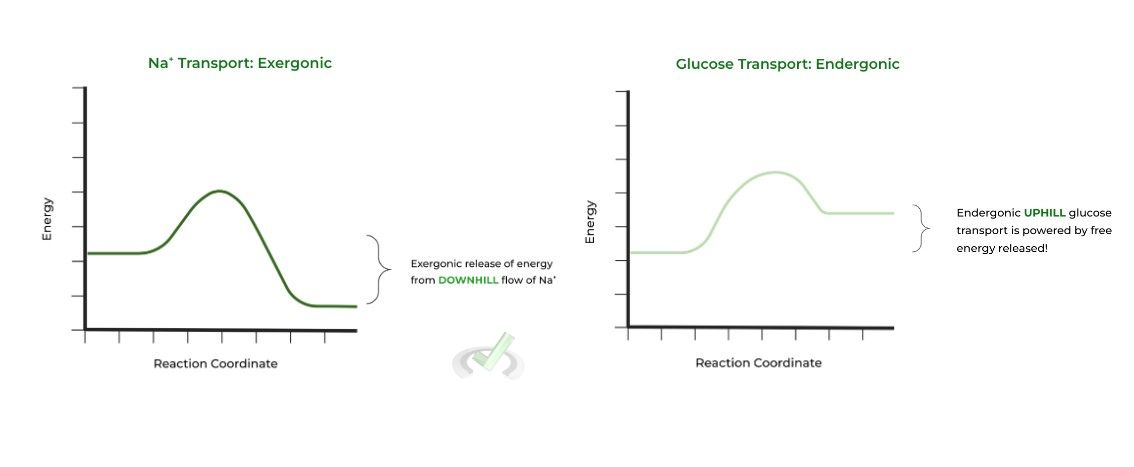
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
The membrane transport of proteins can be categorized in 2 main ways: 1) passive transport and 2) active transport
A. Passive Transport (High to Low) – NO ENERGY REQUIRED
The exergonic, spontaneous movement of substances DOWN their concentration gradient. It can be further subdivided into simple diffusion (no protein required) and facilitated diffusion (protein required).
I. Osmosis
A special type of passive transport which involves the movement of water molecules to areas of high solute concentration (i.e. high osmotic pressure/gradient)!
i. Hypertonic: Net EXIT of water from cell into the extracellular space ⇒ Results in shriveled cell
ii. Hypotonic: Net ENTRY of water to cell from extracellular space ⇒ Results in swelled cell
iii. Isotonic: EQUAL net exit and entry of water in cell ⇒ Cell shape is maintainedB. Active Transport (Low to High) – ENERGY REQUIRED
The endergonic, non spontaneous movement of substances UP, AGAINST their concentration gradient. Further subclassed into primary (1˚) & secondary (2˚) active transport, differing in energy source.
I. Primary (1˚) Transport
Involves the DIRECT use of an energy source, often in the form of ATP. Think of the Na+/K+ ATPases pump as an example! Likewise, many 1˚ proteins will have dual transport and enzyme function!
II. Secondary (2˚) Transport – Coupled Transport
Involves the INDIRECT use of an energy source, which comes from the free energy release from the flow of another substance down its concentration gradient.
The exergonic release of energy from the downhill flow powers the endergonic transport of another substance up, against its concentration gradient!
Transport proteins can also transport 2 particles at the same time! Symporters transport 2 particles in the same direction while antiporters move them in opposite directions!.C. Vesicular Transport – Exocytosis, Endocytosis, Pinocytosis
Involves the creation or fusion of vesicles with the cell membrane! The vesicles are also enveloped with a phospholipid bilayer membrane, which allows for vesicle creation or fusion.
i. Exocytosis: Exiting of substances from cell
ii. Endocytosis: Entering of substance into cells
iii. Pinocytosis: Entering of fluid into cellsV. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
A cell is placed in a hypertonic environment. Which of the following displays how the concentration, in mOsm/kg, in the extracellular space would change?
A. 700 mOsm/kg ⇒ 1000 mOsM/kg
B. 800 mOsm/kg ⇒ 360 mOsM/kg
C. 300 mOsm/kg ⇒ 300 mOsM/kg
D. 600 mOsm/kg ⇒ 900 mOsM/kg
Ans. B
- Because the cell is placed into a hypertonic solution, the water will EXIT and flow out of the cell because the solution is much more concentrated in solutes compared to the inside.
- As such, water will move into the extracellular space and dilute the concentration, which is why the concentration should decrease!
Sample Practice Question 2
In order to study the 2˚ active transport of a particle in vitro, researchers design a membrane embedded with the transport protein in a dish and add the 2 particles involved in the 2˚ active transport as well as ATP. What will happen to the concentration of ATP?
A. The concentration will decrease.
B. The concentration will increase.
C. The concentration will decrease first, then increase.
D. The concentration will remain constant.
Ans.D
- Recall that although secondary active transport involves the use of energy, this energy source is INDIRECT and DOES NOT require the use of ATP as a direct source. Instead, this energy is acquired from the free energy released from the one particle that flows DOWN its concentration gradient.
- As such, the amount of ATP should remain constant, as none will be hydrolyzed!



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























