I. What is the Pyruvate Dehydrogenase Complex?
If you’re coming from our “Oxidative Phosphorylation: Fundamentals and Key Terms” article, welcome back! As you saw in the previous article, we primarily covered the surface level ideas and concepts; now, it’s time to take them apart one by one!
This is the first step succeeding glycolysis and aerobic conditions and is relatively one of the more simple ones compared to the other topics mentioned in the previous article! Consisting of 3 main and 2 regulatory enzymes, we’ll try to explain them as simply as possible!
As a relatively not so high yield content, we’ll try to focus on the crucial, must know components! Our goal is to hopefully cover the most important, high yield topics and tie in how those concepts translate and overlap into other topics!
II. Pyruvate Dehydrogenase Complex
As always, let’s begin with a quick overview of the pyruvate dehydrogenase complex just to quickly orient ourselves!
A. Acetyl-CoA Production via PDH Complex
The pyruvate dehydrogenase complex is composed of 3 main and 2 regulatory enzymes which oxidizes pyruvate to acetyl-CoA, which can go into the citric acid cycle or fatty acid synthesis! Below is a figure you’ve already encountered before!
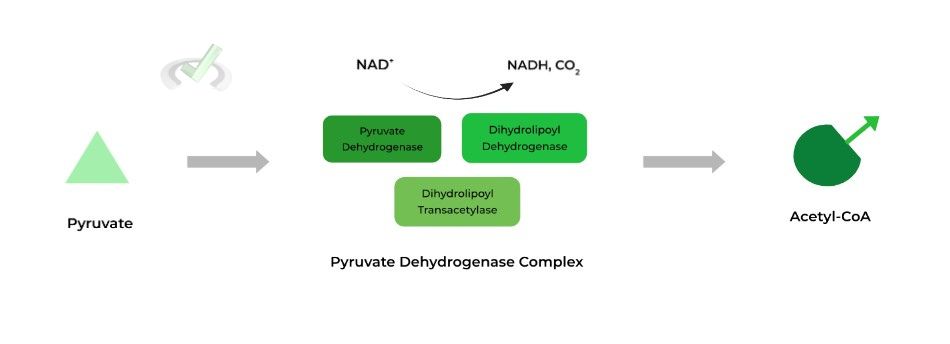
Note that for the article, the number of products is PER 1 PYRUVATE. Glycolysis produces 2 pyruvate molecules so make sure you also account for the other pyruvate when counting the number of products (such as NADH).
B. Complex Components: Main Enzymes
There are 3 main enzymes involved in the complex: 1) pyruvate dehydrogenase, 2) dihydrolipoyl transacetylase, and 3) dihydrolipoyl dehydrogenase. Let’s take a look at their roles!
I. Pyruvate Dehydrogenase
This enzyme catalyzes the initial oxidation of pyruvate, releasing a CO2 molecule as a by-product.
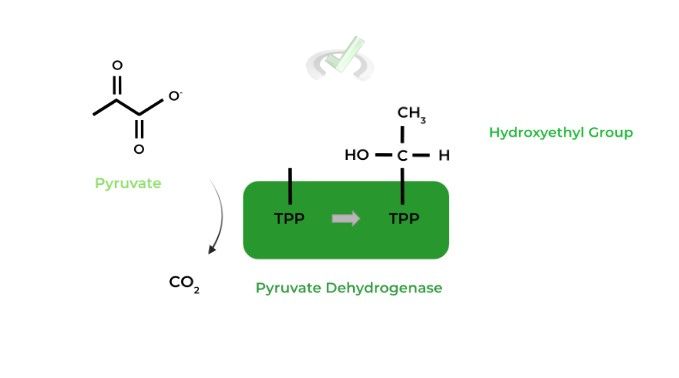
Thiamine pyrophosphate (TPP) is an important coenzyme located in the active site and binds the now oxidized pyruvate, which now exists as a hydroxyethyl group, as shown above.
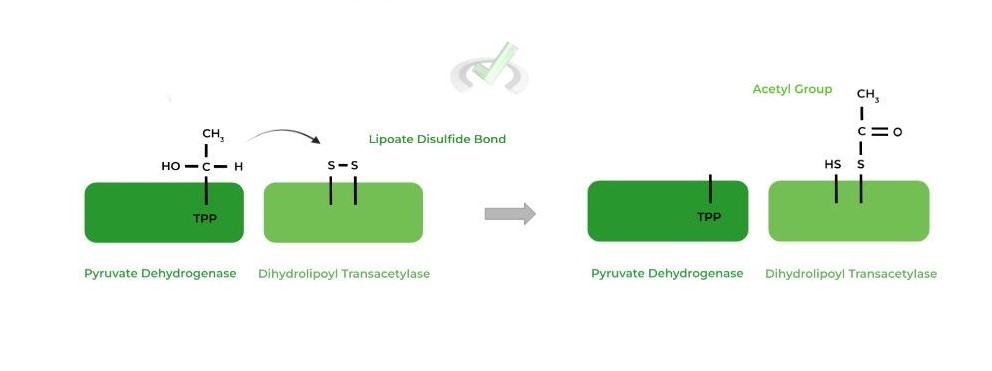
II. Dihydrolipoyl Transacetylase
While we don’t want to pick favorites, we believe this is the most important step of the PDH complex as it transfers the hydroxyethyl group to a coenzyme A to produce acetyl-CoA!
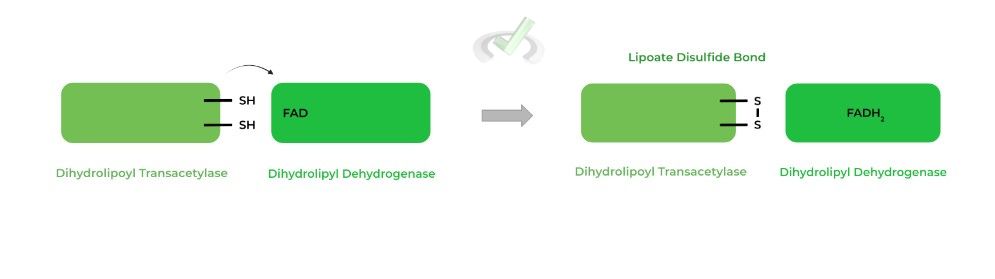
The TPP bound hydroxyethyl group transfers and reduces the disulfide bond on the active site’s lipoate group, while also subsequently being oxidized to an acetyl group.
The acetyl group is then transferred to an incoming coenzyme A (CoA) molecule, generating the main product of acetyl-CoA! Additionally, the other sulfur atom becomes reduced.
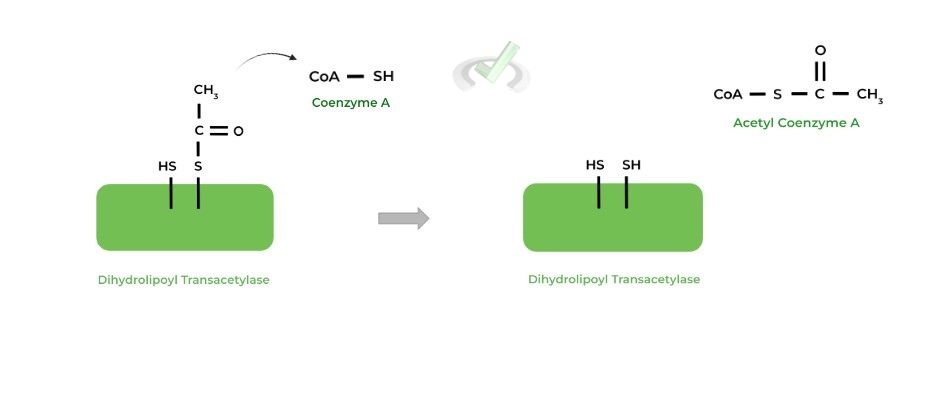
III. Dihydrolipoyl Dehydrogenase
This enzyme catalyzes the oxidation of the reduced sulfhydryl groups to the disulfide bonds from the previous enzyme so as to restart the cycle over again. This occurs via transferring the electrons from the -SH groups to FAD, reducing it to FADH2.
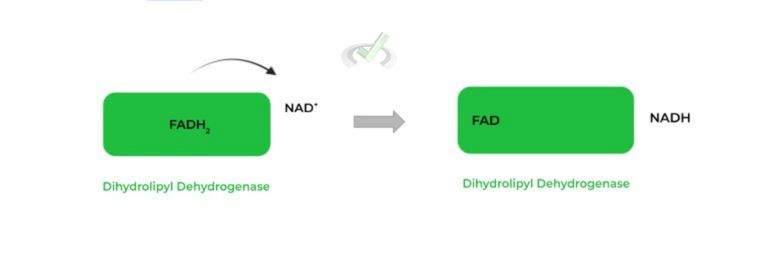
One last transfer of electrons occurs, where FADH2 is oxidized back to FAD so as to reduce NAD+ to NADH and restart the process.
C. Complex Components: Regulatory Enzymes
The big thing about these enzymes is that they function opposite to their general rule of thumb, such that the kinase actually causes INHIBITION and the phosphatase initiates ACTIVATION!
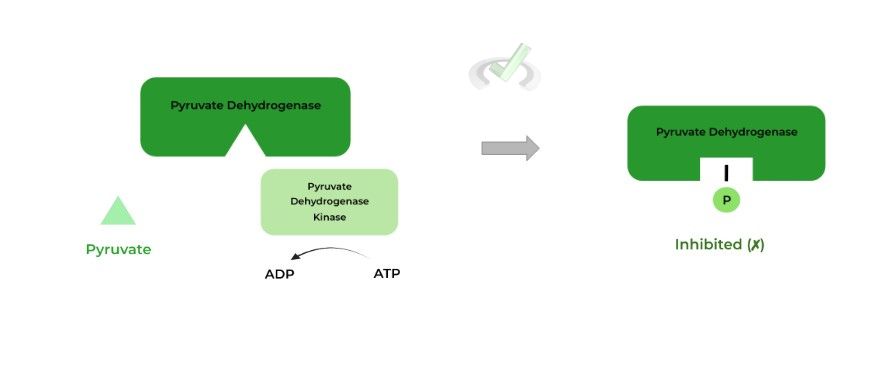
I. Pyruvate Dehydrogenase Kinase
The PDH kinase actually inhibits pyruvate dehydrogenase (as opposed to activating it)! The phosphorylation of the enzyme’s active site causes steric hindrance and unfavorable electrostatic interactions to where there’s an inactivating conformational change.
II. Pyruvate Dehydrogenase Phosphatase
As indicated by its name, the PDH phosphatase will actually activate pyruvate dehydrogenase as it works to remove the PDH kinase added phosphate group.
By removal of the phosphate group, the pyruvate dehydrogenase active site is no longer hindered and is now activated.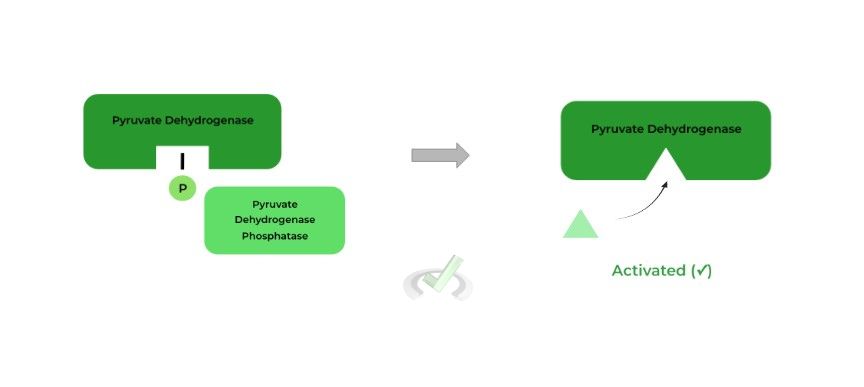
III. Bridge/Overlap
Did that name or term “disulfide” ring a bell? Let’s throw it back to a little review of amino acid residues and even include a little bit of oxidation reduction chemistry to get a better understanding of the PDH complex!
I. Disulfide Bonds
Recall that disulfide bonds form when 2 thiol/sulfhydryl (-SH) groups are oxidized and form a bond with each other. This is very common in 3˚ and 4˚ protein structures such as those seen in antibodies and can only be formed by cysteine residues!
The disulfide bonds present in dihydrolipoyl transacetylase are very similar! Though they aren’t made of cysteine residues, the lipoate groups contain 2 thiol groups, which allows for the disulfide bond formation!
II. Oxidized and Reduced States
Disulfide bonds represent the sulfur in their oxidized state as they LOSE electrons. Contrarily, the thiol groups (-SH) represent the sulfurs in their reduced state as they GAIN electrons.
A good, general rule of thumb to have a better visual of reduced and oxidized states is to see how many hydrogen atoms are attached!
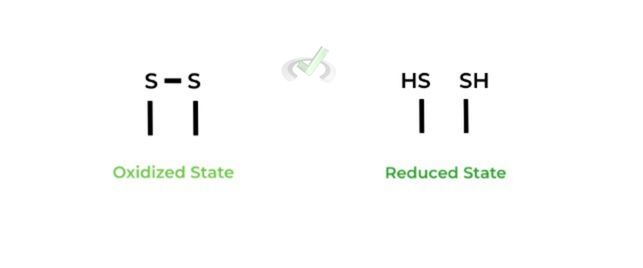
Notice how in the reduced state, the sulfurs are in the form of a thiol where a hydrogen is bounded! Hydrogens and hydrides are great electron donors and reducing agents because they donate their electrons and usually will have a lower electron affinity!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Acetyl-CoA Production via PDH Complex
The pyruvate dehydrogenase (PDH) complex allows for the further oxidation of pyruvate to produce NADH and acetyl-CoA, with the latter being able to go into the citric acid cycle to be used for fatty acid synthesis.
B. Complex Components: Main Enzymes
The 3 main enzymes of the PDH complex are: 1) pyruvate dehydrogenase, 2) dihydrolipoyl transacetylase, and 3) dihydrolipoyl dehydrogenase.
I. Pyruvate Dehydrogenase
Contains a thiamine pyrophosphate (TPP) coenzyme in its active site; catalyzes the oxidation of pyruvate to a hydroxyethyl group, also releasing CO2 as a by-product.
The hydroxyethyl group is bound to TPP, which will then be transferred to dihydrolipoyl transacetylase in the next step.
II. Dihydrolipoyl Transacetylase
Contains lipoate groups in its active site; first catalyzes the transfer of the TPP bound hydroxyethyl group which oxidizes the lipoate’s group disulfide bond, while also oxidizing the hydroxyethyl group to an acetyl group.
The enzyme then catalyzes the transfer of the acetyl group to an incoming CoA, producing acetyl-CoA, also reducing the remaining sulfur to a thiol group.III. Dihydrolipoyl Dehydrogenase
Catalyzes first the oxidation of the thiol groups back to the disulfide bond by transferring their electrons to FAD, reducing it to FADH2.
Another transfer of electrons occurs, where FADH2 is oxidized back to FAD to restart the cycle by transferring its electrons to NAD+, generating NADH.C. Complex Components: Regulatory Enzymes
These enzymes behave opposite to their rule of thumb, where the kinase actually INHIBITS pyruvate dehydrogenase and the phosphatase ACTIVATES pyruvate dehydrogenase.
I. Pyruvate Dehydrogenase Kinase
Inhibits pyruvate dehydrogenase via phosphorylation; this is due to the steric hindrance & unfavorable electrostatic interactions caused by the phosphate group results in inactivating conformational change.
II. Pyruvate Dehydrogenase Phosphatase
Activates pyruvate dehydrogenase by removing the inactivating phosphate group placed by pyruvate dehydrogenase kinase!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
After 1 round of glycolysis and the pyruvate dehydrogenase complex, how many NADHs are produced?
A. 2
B. 4
C. 6
D. 8
Ans. B
In one round of glycolysis, 2 NADH are produced in step 6 when the 2 glyceraldehyde-3-phosphates are oxidized to 1,3-bisphosphoglycerate. Glycolysis will also result in the production of 2 pyruvates.
One round of the pyruvate dehydrogenase complex produces 1 NADH through dihydrolipoyl dehydrogenase. However, recall that 2 pyruvates are generated at the end of glycolysis. Thus, 2 NADH molecules are produced via the PDH complex for a total of 4 NADH.
Sample Practice Question 2:
The PDH complex and its associated regulatory enzymes are placed in an in vitro setting containing no ATP. Which of the following best describes the activity of the PDH complex and/or the enzymes?
A. The PDH complex will be constantly activated
B. The PDH complex will be constantly inhibited
C. The pyruvate dehydrogenase kinase enzyme will be constantly activated.
D. The pyruvate dehydrogenase enzyme will be constantly inhibited.
Ans. A
The pyruvate dehydrogenase kinase requires ATP for phosphorylation and inhibition of PDH and the complex as a whole. Because no ATP is present, the pyruvate dehydrogenase won’t be inhibited and will remain active, as will the complex as a whole.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























