I. What are the Commonly Tested Sugars?
As indicated by the name, these sugars are probably the ones that are most commonly tested on the MCAT! While how this topic will be addressed on the MCAT might vary, it’s always beneficial to have a good insight on the structure of the molecule when encountering problems!
Some questions might outright test your ability to recall and memorize the structure of a certain sugar but more often than not, it can be used as a beneficial tool to picture or at least get a general idea of the sugar when it’s mentioned in a passage, for example.
One thing we preach here at MCAT Mastery is to find commonalities between molecules and see how that can aid your memorization and review! We’ll highlight some patterns that may help you all in memorizing these structures!
II. Monosaccharides, Disaccharides, and Polysaccharides
As we’ve discussed previously, we’ll break down the commonly tested carbohydrates into 1) monosaccharides, 2) disaccharides, and 3) polysaccharides.
A. Monosaccharides
Recall that monosaccharides are the monomeric subunits that allow for the formation of disaccharides and polysaccharides.
Some commonly tested sugars include glucose, fructose, and galactose, as these will form the building blocks of disaccharides and polysaccharides as we’ll see later.
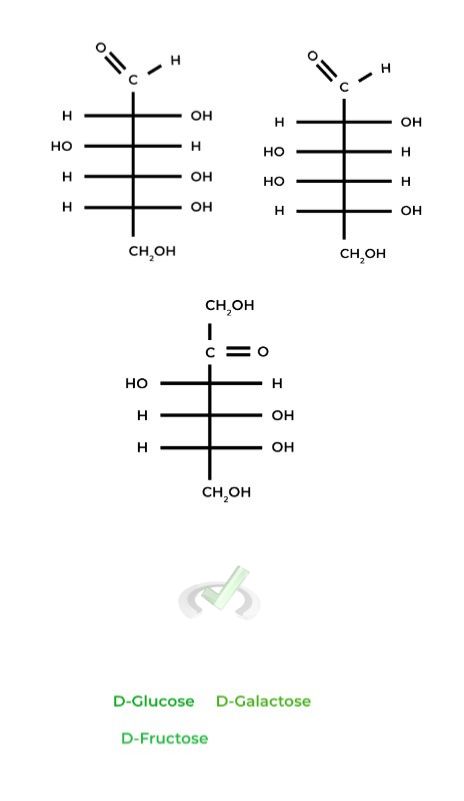
Note that all the sugars are in the D-enantiomeric form. Try and find commonalities between the sugar molecules to make memorization easier!
For example, you may have noticed that D-glucose & D-galactose are essentially the same structure, differing only at the C4 carbon.
Additionally, don’t forget that the deoxyribose and ribose of nucleic acids are also sugars! Look back to our other nucleic acid articles for a quick review of the structures!
A. Disaccharides
As implied by its name, these carbohydrates are composed of 2 monosaccharides subunits. Similarly, some of the most commonly tested include sucrose, lactose, and maltose.
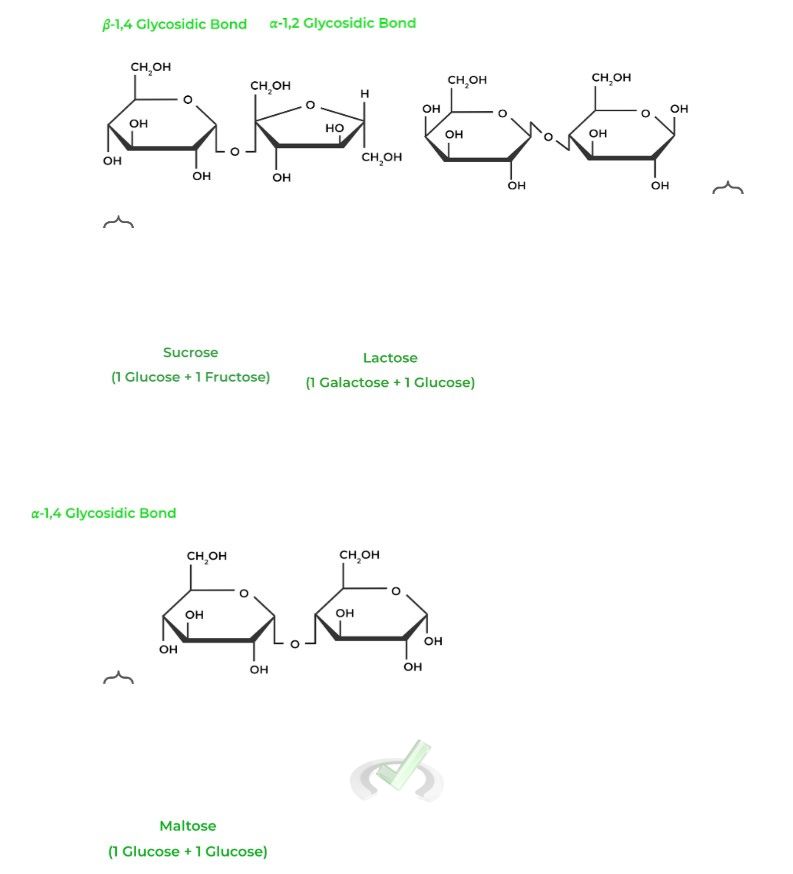
In addition to the monosaccharides that make up the disaccharides, we’ve also included the type of glycosidic bond which connects the carbohydrates together!
The numbers in front simply refer to which carbons are being connected by the bond while the 𝜶 or 𝜷 term refers to whether the glycosidic bond is placed below or above the Hawthorne projection.
For example, the galactose C1 and glucose C4 in lactose are connected by a 𝜷-glycosidic bond, meaning that the glycosidic bond is placed ABOVE, similar to the rules for anomers!
C. Polysaccharides
As implied by the name, polysaccharides are composed of many monosaccharides subunits. Similarly, some of the ones that you’ll most likely encounter on the MCAT are glycogen, starch, and cellulose.
Glycogen is the most likely polysaccharide you’ll encounter on the test as it serves as a storage method for glucose and is involved in many metabolic reactions!
In the context of this article, know that glycogen is a polysaccharide composed of many glucose monomers linked LINEARLY by 𝛂-1,4 glycosidic bonds and are BRANCHED via 𝛂-1,6 glycosidic bonds.
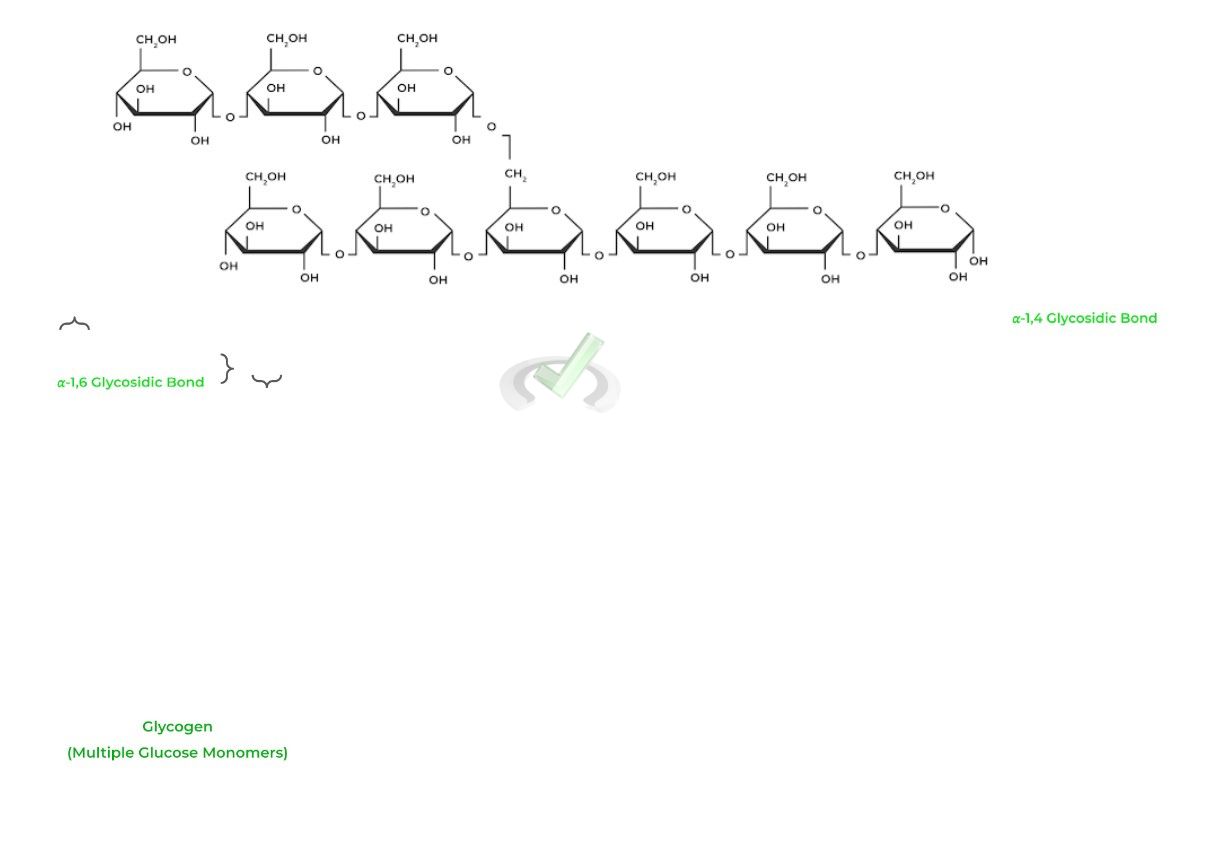
For further information on glycogen’s function and synthesis, refer to our glycogen articles in the carbohydrate metabolism section for a better understanding!
Starch is actually very similar to glycogen, also being composed of 𝛂-1,4 glycosidic bonds, but does not include the branched 𝛂-1,6 glycosidic bonds.
The main difference between the 2 is that glycogen is used as an energy storage mechanism while starch is an exogenous energy source coming from stuff like plants and potatoes!
Cellulose is the main structural component of plant cell walls composed of 𝜷-1,4 glycosidic bonds. However, our bodies lack the enzyme, specifically cellulase, that allows for the hydrolysis of the 𝜷-1,4 glycosidic bonds in cellulose, preventing digestion.
This is why plants are a good source of fiber in order to help with constipation! The osmotic pressure of cellulose draws water into the lumen of the intestines, softening the stool.
III. Bridge/Overlap
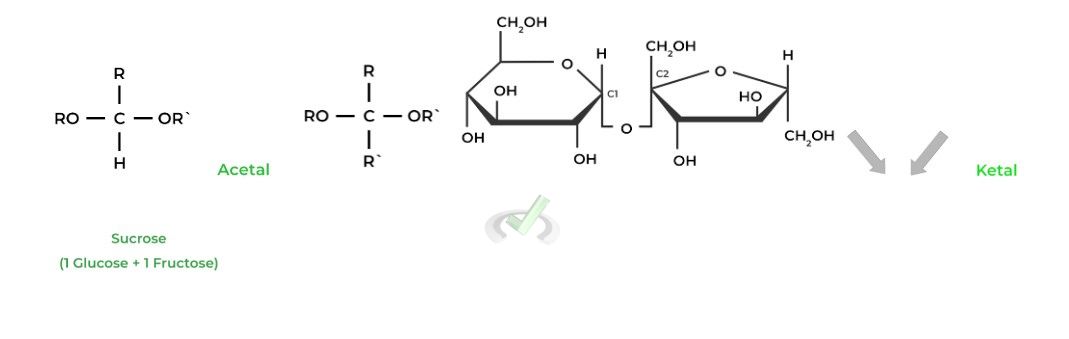
Did you by chance notice something about the disaccharides and the bonds they form? If you’ve by chance reviewed some of the organic chemistry sections, you’re right to say that an acetal or ketal group was formed! Let’s get a quick review on these basic organic molecules!
I. Acetals and Ketals in Carbohydrates
Acetals and ketals are derived from their precursor hemiacetals and hemiketals, respectively. Both will be formed where another -OR substitutes the remaining -OH group.
A great example of this is in sucrose where the glucose’s C1 anomeric carbon becomes an acetal and fructose’s C2 anomeric carbon becomes a ketal!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Monosaccharides
These are essentially the monomeric subunits that form the basis of disaccharides and polysaccharides. The most common ones you’ll need to know about are glucose, fructose, and galactose. Don’t forget also that deoxyribose and ribose of nucleotides are also sugars!
B. Disaccharides
As indicated by the name, disaccharides are composed of 2 monosaccharides subunits. The most common ones you’ll need to know are sucrose, lactose, and maltose.
Sucrose is composed of glucose and fructose connected by an 𝛂-1,2 glycosidic bond. Lactose is composed of glucose and galactose connected by a 𝜷-1,4 glycosidic bonds. Maltose is composed 2 glucoses connected by an 𝛂-1,4 glycosidic bond.
C. Polysaccharides
These are composed of multiple monosaccharide monomer subunits. THe most common ones you’ll need to know about are glycogen, starch, and cellulose.
Glycogen serves as an energy storage mechanism in the body and is composed of multiple glucose monomers connected LINEARLY by 𝛂-1,4 glycosidic bonds and BRANCHED via 𝛂-1,6 glycosidic bonds.
Starch is an exogenous energy source which like glycogen also has 𝛂-1,4 glycosidic bond but no 𝛂-1,6 glycosidic bonds. Cellulose is a structural component for the plant cell wall and is connected by 𝜷-1,4 glycosidic bonds.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Which of the following can be considered a homodisaccharide? (Hint: Look at the prefix!)
A. Sucrose
B. Lactose
C. Fructose
D. Maltose
Ans. D
The “-homo” and “-hetero” prefixes come up often meaning “same” and “different” respectively. We can deduce that a monosaccharide is a disaccharide that contains 2 of the same monosaccharides, which best describes maltose as it’s composed of 2 glucose molecules.
Sample Practice Question 2
Which of the following best describes the relationship between D-glucose and D-galactose?
A. Epimers
B. Anomers
C. Enantiomers
D. Ketoses
Ans. A
Recall that epimers are pairs of stereoisomers that only differ in one stereocenter. In this case, glucose and galactose only differ in the C4 carbons! Note that they cannot be anomers as they don’t different in the hydroxyl position on the anomeric carbon!



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























