I. What is the Structural Description of Carbohydrates?
To eat or not to eat? Do I add more or less to my diet? While we’re not expert nutritionists or dieticians here at MCAT Mastery, we can definitely say with confidence that carbohydrates are crucial and essential part of your diet and physiological functioning!
As you’ll see in the carbohydrate metabolism section, these simple and complex sugars are crucial in being a source of energy that the body can readily rely on in order to power the various cellular processes that keep bodies functioning and in shape!
One great thing about studying and reviewing this section is that you’ll without a doubt see a great and clear overlap between carbohydrate structure and function and some basic organic chemistry principles, a good way also to refresh on some basic ochem fundamentals!
II. Structural Basics of Carbohydrates
Carbohydrates encompass all sugars, both simple and complex sugars. Simple sugars include monosaccharides and disaccharides while complex sugars include oligosaccharides and polysaccharides. Let’s first focus on the simple, monosaccharide sugar structure before we start building further!
A. Monosaccharide Structure
On a molecular level, simple monosaccharide sugars will follow the chemical formula CnH2nOn. However, this won’t hold true for disaccharides and complex sugars due to water loss during bond formation.
These simple sugars are categorized into 2 main groups: 1) aldoses, if they contain an aldehyde group, and 2) ketoses, if they contain a ketone group. Here are a couple of common examples!
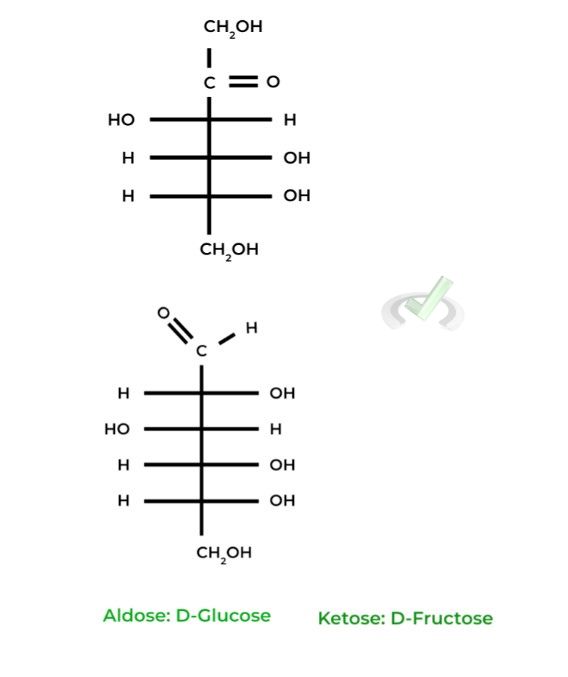
Furthermore, there’s a lot more that goes into describing carbohydrate structure including carbon numbering, structure representation, and stereochemistry (agh!). Though a little intimidating at first, let’s take everything step by step!
I. Carbon Numbering
The numbering of the carbons becomes particularly important when talking about Hawthorne projections of sugars, as we’ll discuss later. Luckily, the numbering of the carbons follows the same rules as in organic chemistry!
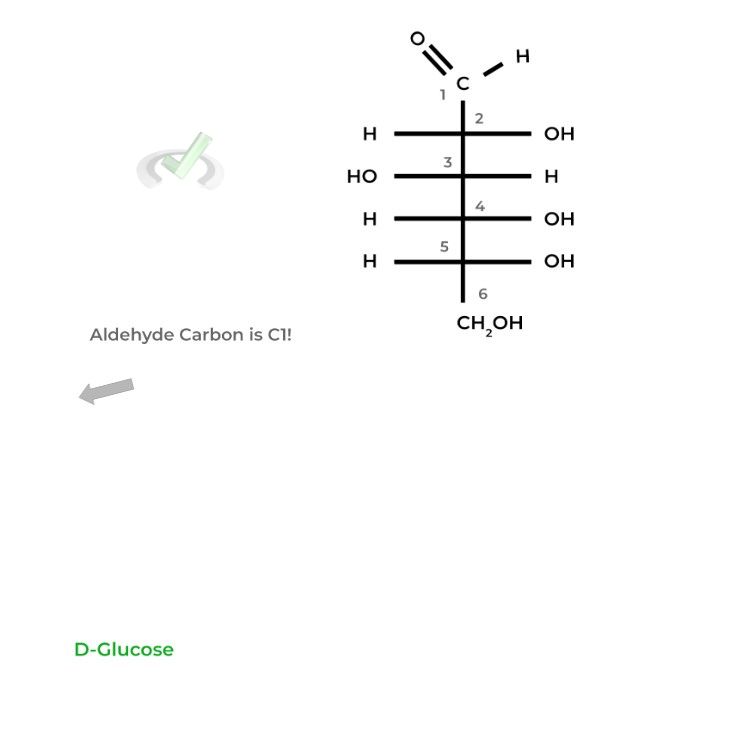
For aldoses, the carbon containing the aldehyde group is labeled carbon 1 (C1) as it’s the most oxidized, followed by the rest of the carbons.
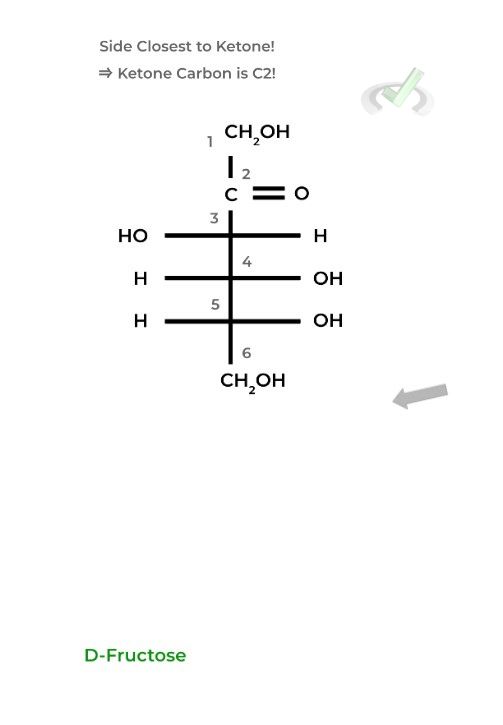
For ketoses, start the numbering from the side closest to the ketone carbon as it needs to have the lowest number possible being the most oxidized carbon.
II. Structure Representation
There are 2 main ways that the simple sugars can be projected and represented on paper: the Fischer Projection and the Hawthorne Projection, which are used for depicting sugars in a planar and cyclic way, respectively,
The D-glucose and D-fructose shown above are examples of Fisher projections, where the horizontal lines represent wedges and the vertical lines represent dashes.
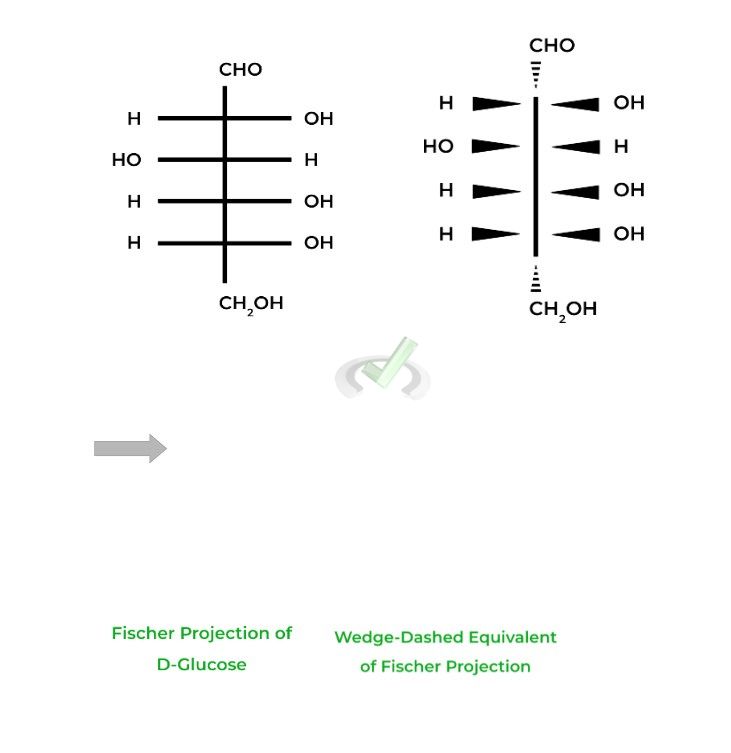
Don’t worry about trying to visualize it in 3D space. What’s important to know about Fischer Projections is that they are a great way to quickly identify differences in stereochemistry, especially in enantiomers.
The Hawthorne Projection depicts a cyclic representation of the sugars as pyranoses, 6 membered rings, or furanoses, 5 carbon rings. This is accomplished by a nucleophilic attack by a hydroxyl group on the highest numbered chiral carbon.
Additionally, the carbon that bears the aldehyde or ketone, in this case C1, is termed the anomeric carbon which is important in the next section!
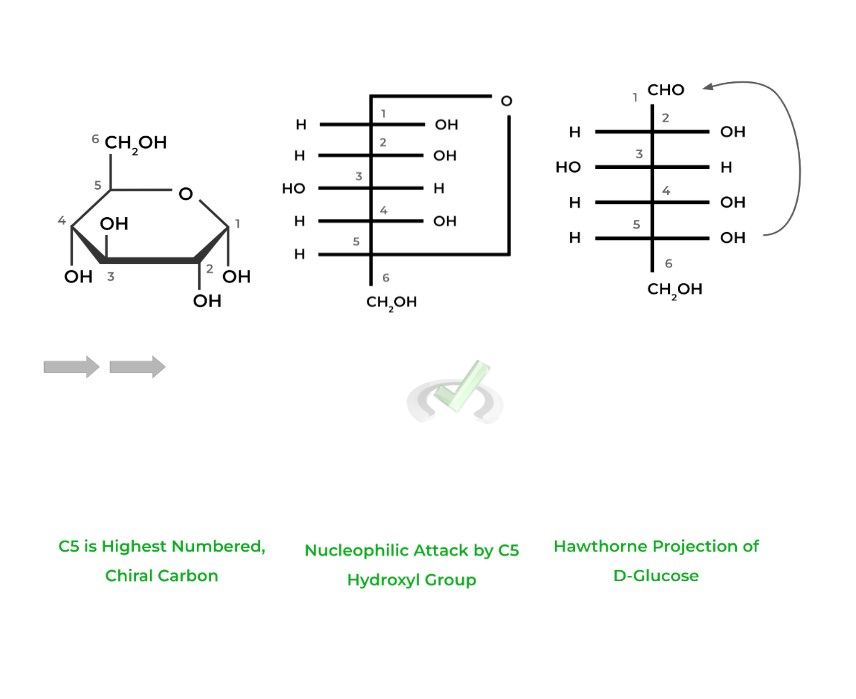
III. Stereochemistry
We know this topic sounds scary at first but there are really only 2 stereochemical topics that need to be applied in carbohydrate biochemistry: enantiomers in D and L Fisher projections. and anomeric epimers in Hawthorne projections.
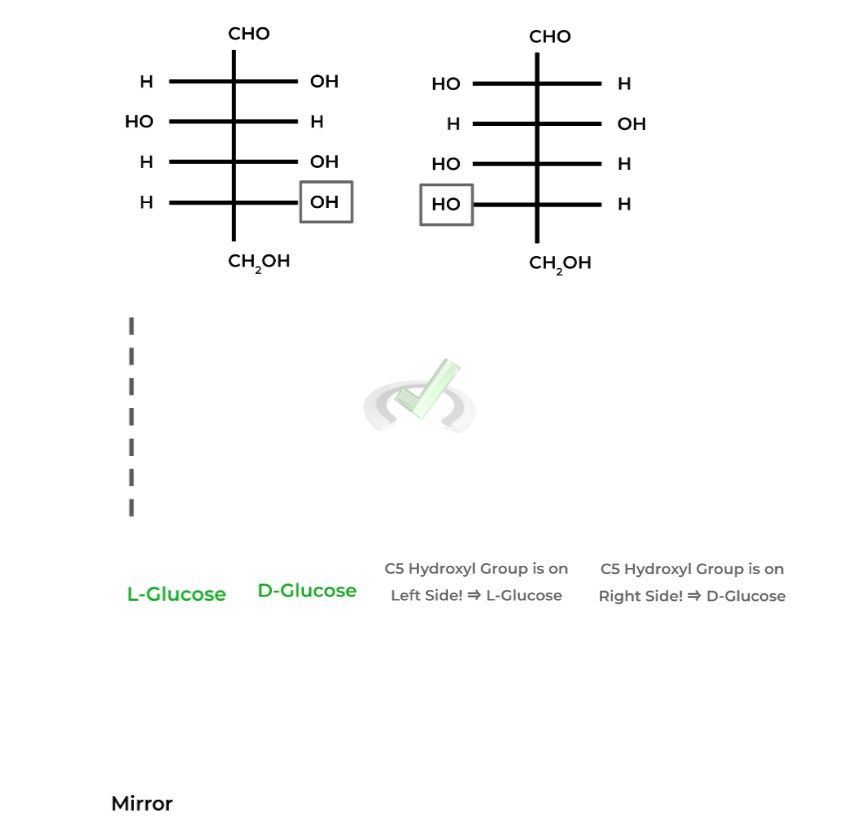
The “D” in front of D-Glucose refers to 1 enantiomer of the pair with the other being L-Glucose, as they are nonsuperimposable images of one another.
To differentiate between the two, D-Glucose will have the hydroxyl group on
the highest numbered chiral carbon ON THE RIGHT in its Fischer projection while L-Glucose will be ON THE LEFT.
Anomers are actually a subclass of epimers, which are stereoisomers that differ only in the position of 1 stereocenter. This is depicted in the Hawthorne projections, where the glucose molecules only differ in the position of the anomeric carbon!
The alpha anomer of D-glucose has the anomeric hydroxyl group facing DOWN while its facing UP in the beta anomer.
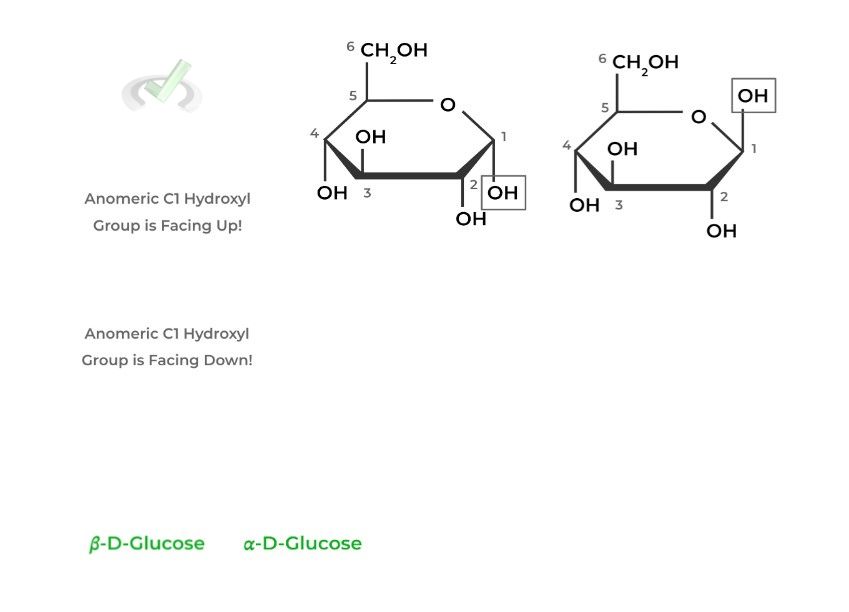
III. Bridge/Overlap
As always, with any organic reaction that’s mentioned in chemical and biochemical processes, it’s always helpful to get a brief overview of the reaction mechanism to get a better idea of what’s going on. Remember that for the MCAT, it doesn’t have to be the nitty-gritty! Just enough to get a clearer picture!
I. Nucleophilic Attack Mechanism in Sugar Cyclization
You’ve probably seen a similar reaction mechanism when discussing the addition of new nucleotides to an existing DNA strand. Essentially the reaction is the same but just with different reactants!
We still have a nucleophilic hydroxyl group as the attacking reactant. However, now it attacks the carbonyl carbon of the aldehyde or ketone located on the ring, an intramolecular reaction resulting in cyclization.
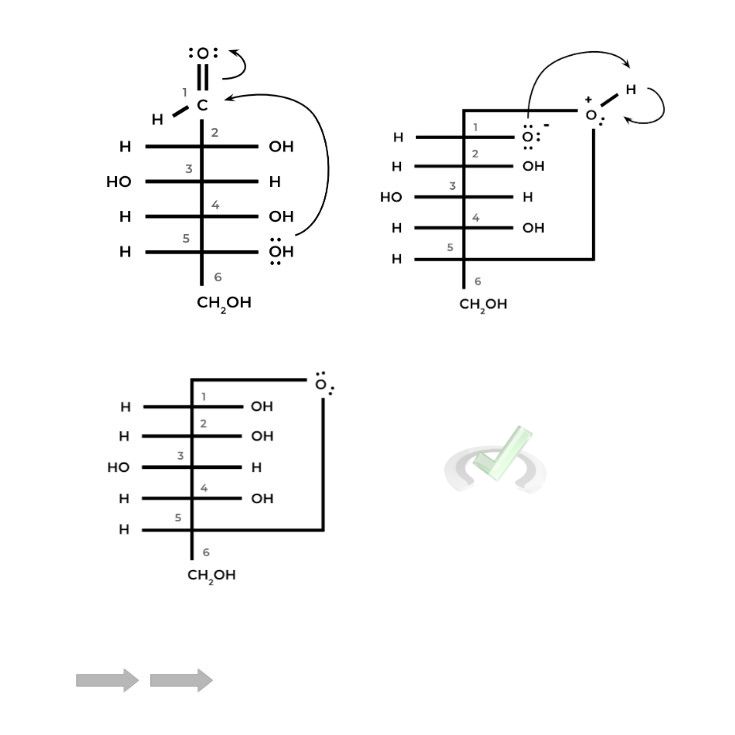
This is actually a variation of some reactions we’ve encountered, specifically hemiacetal and hemiketal formation if the sugar is an aldose and ketose, respectively.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Monosaccharide Structure
On the molecular level, monosaccharides follow the chemical formula, CnH2nOn, which doesn’t necessarily hold true for disaccharides and complex sugars due to the loss of a water molecule during bond formation.
Monosaccharides are broken down into 1) aldoses, if they contain an aldehyde, and 2) ketoses, if they have a ketone group.
I. Carbon Numbering
Luckily, the numbering of carbohydrates and carbons follows the same rules as organic chemistry!
For aldoses, the carbon containing the aldehyde will be labeled carbon 1 (C1) as it’s the most oxidized carbon followed by the rest of the carbons.
For ketoses, start numbering at the side closest to the ketone, as the carbon ketone must have the lowest possible number as it’s also the most oxidized carbon.
II. Structure Representation
There are 2 ways the carbohydrates are mainly depicted: 1) Fischer Projection, for a planar view, and 2) Hawthorne Projection, for a cyclic view.
Fischer projections allow for a quick identification of differences in stereochemistry between carbohydrates, especially in enantiomers.
The horizontal lines represent wedges while the vertical lines represent dashes.
Hawthorne projections depict pyranoses, 6 membered rings, and furanoses, 5 membered rings.
These result from the nucleophilic attack of the hydroxyl group on the highest numbered chiral carbon.
III. Stereochemistry
There are really only 2 must know applications of stereochemistry in carbohydrate biochemistry: enantiomers in D and L Fisher projections and anomeric epimers in Hawthorne projections.
Carbohydrate sugars come in enantiomeric pairs, indicated by the D or L in front of the sugar name.
The D indicates that the hydroxyl group on the highest numbered chiral carbon is ON THE RIGHT of the FIsher projection while the L indicates that it’s ON THE LEFT.
Anomers are a subclass of epimers, which are stereoisomers which different at ONLY 1 stereogenic center, as depicted in the Hawthorne projections where sugars differ only in the anomeric carbon, which is the carbon containing the aldehyde or ketone..
The alpha anomer of a sugar has the anomeric hydroxyl group facing DOWN while its facing UP in the beta anomer!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Shown below is the Fischer Projection of the sugar galactose. Which enantiomeric pair of galactose is this and what is the best classification for this sugar?
A. L-Galactose; Ketose
B. L-Galactose; Aldose
C. D-Galactose; Ketose
D. D-Galactose; Aldose
Ans. B
First, we see that the highest numbered chiral carbon’s hydroxyl group is placed ON THE LEFT of the Fischer Projection, indicating this is the L-enantiomer. Furthermore, we see an aldehyde group on galactose, indicating that it should be classified as an aldose.
Sample Practice Question 2
T/F: Aldoses will always have the aldehyde carbon labeled as carbon 1 (C1).
Ans. T
Remember that aldehydes always have to be at the end of the carbohydrate chain as the connection to the hydrogen atom triggers the end of the chain. As such, the aldehyde will always be at the end and always be labeled as C1 because it’s the most oxidized carbon.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























