I. What is DNA Structure and Function?
Before tackling the big topics such as replication, transcription, and even mitosis, it’s important to get our feet wet with the basics of DNA. It just goes to show the importance of DNA, whether it’s studying 6th basic biology or the life-changing MCAT exam (which we know you’ll all crush!)
In this article, we’ll go over everything you all need to know about DNA before going further into more of the complex topics. From covering its double helix structure to the Chargaff’s rule, the basic foundations laid out in the article will hopefully make studying other DNA topics much easier!
We cannot stress enough the importance of DNA during MCAT prep, as there are so many overlaps in terms of study content that can be made! They can relate to more similar topics such as cell division & viral replication, but can also relate to general chemistry topics such as hydrogen bond formation!
II. Structure and Function of DNA
We mentioned in another article that DNA primarily exists in the cell as a double-stranded molecule. Let’s dive into the importance of its double-strand structure.
A. The Double Helix Structure
Probably the most identifiable feature of DNA’s structure is its double helix. This allows for the formation of a helical structure, where the DNA molecule can turn into a spiral shape as shown below:
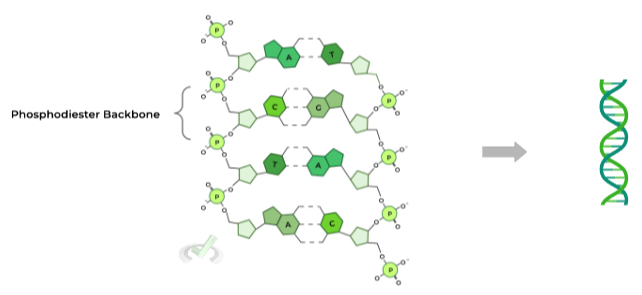
Another important structural component is the phosphodiester backbone, constructed with the phosphate groups connecting the nucleotides. The phosphate composition of the DNA backbone confers a NEGATIVE, overall charge on the DNA.
Within the backbone, a phosphate group connects the C5 carbon of one nucleotide to the C3 carbon of another nucleotide, which is important when talking about DNA’s antiparallel orientation!
I. DNA Helix Orientation
Some important results from DNA’s double helix formation are its complementary base pairing between the strands and the antiparallel orientation.
There are 2 important aspects to grasp before understanding the antiparallel orientation of DNA: 1) the DNA molecule is double stranded and 2) the phosphate groups connect nucleotides via the C5 and the C3 carbon.
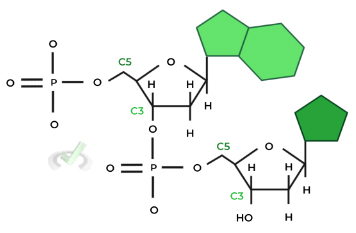
As shown in the diagram above, the top most carbon is the C5 carbon while the bottom most carbon is the C3 carbon. Because of this orientation, this is called a 5 to 3 direction for the DNA molecule.
In its most simplest terms, antiparallel orientation refers to the DNA’s strand running in opposite directions.
One strand runs in a 5 to 3 direction starting from the top while the complementary strand runs in a 5 to 3 direction starting from the bottom.
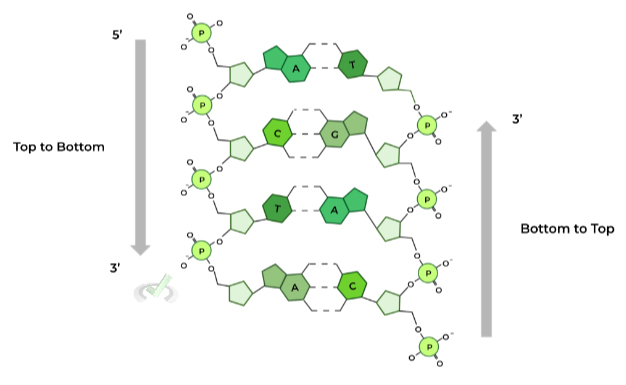
A simple way to think about the antiparallel orientation is that the other strand is “flipped” in relation to the complementary DNA strand!
B. Watson Crick Base Pairing Specificity: A to T and C to G
Another key element of DNA’s structure is the pairing of the nitrogenous bases of the nucleotides. As you’ve seen in the other figures, the bases kinda look like rungs on a ladder when paired together on the double helix.
The rules for Watson-Crick base pairing are quite simple: adenine (A) pairs with thymine (T) while cytosine (C) pairs with guanine (G). Take note at how the base pairs involve one purine base and one pyrimidine base!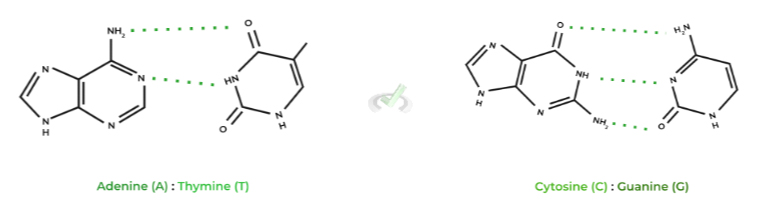
Did you also notice the dotted lines that connect the base pairs? These represent the hydrogen bonds which form the strong interactions between the 2 nucleotides!
I. Denaturation, Reannealing, and Hybridization
The complementary base pairing also forms the basis of the above mentioned processes! These actually come into play when talking about processes such as PCR! Let’s briefly define these terms!
Denaturation simply refers to the separation of the dsDNA into single strands usually via heat while reannealing refers to the recombining of the single strands to reform the dsDNA.
Sometimes, ssDNA from a different source, such as a DNA probe, can bind and hybridize with the denatured DNA, given that it has complementary base pairing!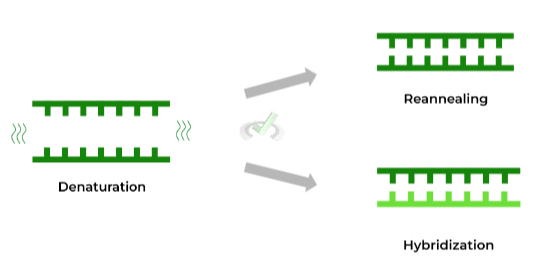
II. Chargaff’s Rule
From this base pair scheme, we can also apply what’s called Chargaff's Rule. In its simplest terms, the rule states: the percentage of amount of adenine (A) must equal thymine (T) and the percentage amount of cytosine (C) must equal guanine (G).
This 1:1 ratio is due to the complementary Watson-Crick base pairing. As such, the individual percentages of the bases that compose a DNA molecule must equal 100%.
Problems with Chargaff’s rule will want you to find the other base percentages when only given one. Here’s a step-by-step strategy to use when encountering these problems!
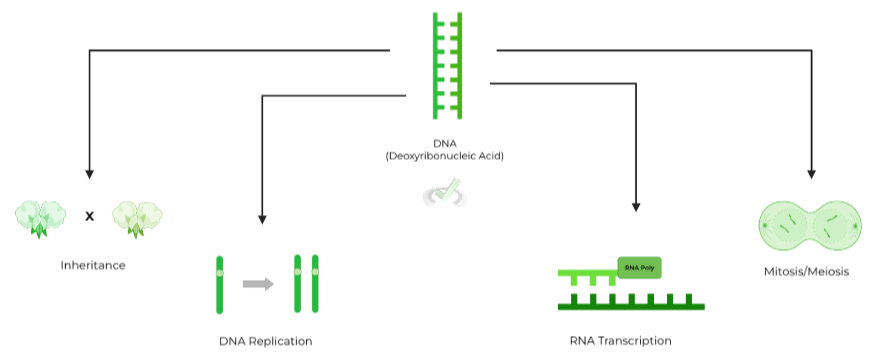
C. The Role of DNA: Genetic Transmission, Inheritance, and Much More
Aside from being what makes you “you” (you’re awesome, btw! 
III. Bridge/Overlap
Remember those dotted lines from the earlier Watson-Crick base pairs? We know that they represent the hydrogen bonds that form between the nitrogenous bases, an important type of intermolecular bond. Let’s briefly review those bonds!
I. Hydrogen Bonds
These bonds can actually be thought of as a special type of dipole-dipole interaction. There are 2 main components in all hydrogen bonds: 1) a hydrogen bond donor and 2) an hydrogen bond acceptor.
The donor consists of a hydrogen atom bonded to either a nitrogen, fluorine, or oxygen atom while an acceptor will either be a nitrogen, fluorine, or oxygen atom.
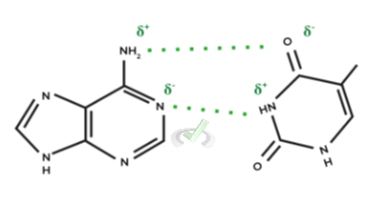
An also important note is that the C-G base pair contains 3 hydrogen bonds while the A-T base pair only contains 2 hydrogen bonds. As such, a DNA molecule with a higher C-G base pair concentration will require a higher temperature in order to denature the DNA strand.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. The Double Helix Structure
The most identifiable feature of the DNA is its double helix, which forms a helical, spiral structure with complementary base pairing between the nucleotides.
DNA also has a phosphodiester backbone, where phosphate groups connect the nucleotides via the C5 and C3 carbons. In addition, the phosphate groups also confer a NEGATIVE, overall charge.I. DNA Helix Orientation
The DNA molecule is oriented where the strands run antiparallel to one another. In its simplest terms, antiparallel orientation refers to the DNA’s strands running in the opposite direction.
One strand runs from the 5’ to 3’ direction starting from the top while the other strand runs 5’ to 3’ starting from the bottom.B. Watson-Crick Base Pairing Specificity: A to T and C to G
The nitrogenous bases on the nucleotides have specific base pairs that form on the DNA double helix. Fortunately the rules are simple: adenine (A) pairs with thymine (T) while cytosine (C) pairs with guanine (G).
I. Denaturation, Reannealing, and Hybridization
The complementary base pairing forms the basis for the above mentioned processes. Denaturation refers to the separation of the dsDNA while reannealing refers to the combining of the separated DNA strands.
A DNA strand from a different source can sometimes bind and hybridize to one of the original strands given it has the correct base pairing.II. Chargaff’s Rule
Corresponding base pairs will have a 1:1 ratio in regards to percentage composition in the DNA molecule. The sum of all the individual base pair percentages will be 100%.
Example:
-18% A = 18%T
-32% C = 32% G
C. The Role of DNA: Genetic Transmission, Inheritance, and Much More!
DNA is involved in many cellular processes including, but not limited to, inheritance, DNA replication, RNA transcription, and mitosis/meiosis. You’ll probably see that most biochemical processes can point directly back to DNA and its involvement in one way or another!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Finish the chart below detailing the nucleotide composition of a DNA molecule.
Nitrogenous Base | Percentage (%) |
|---|---|
A | |
T | |
C | 32 |
G |
Ans.
Nitrogenous Base | Percentage (%) |
|---|---|
A | 18 |
T | 18 |
C | 32 |
G | 32 |
This particular question is testing your ability to apply Chargaff’s rule in order to determine the missing nucleotide base percentage composition. Use the strategy that we’ve outlined earlier in the article to approach this problem!
Sample Practice Question 2
Which of the following is the correct description between the hydrogen bond pairs formed between guanine and cytosine as depicted below?
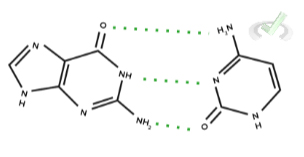
A. Guanine has 2 hydrogen bond donors; Cytosine has 1 hydrogen bond donor.
B. Guanine has 2 hydrogen bond acceptors; Cytosine has 1 hydrogen bond acceptors.
C. Guanine has 1 hydrogen bond donor; Cytosine has 2 hydrogen bond donors.
D. Guanine has 1 hydrogen bond donor; Cytosine has 1 hydrogen bond acceptor.
Ans. A.
A hydrogen bond donor must contain a hydrogen atom bonded to either oxygen, nitrogen, or fluorine (electronegative atoms) while a hydrogen bond acceptor is either an oxygen, nitrogen, or fluorine atom.
Going off of this, we can see that guanine has 2 donors and 1 acceptor while cytosine has 2 acceptors and 1 donor.




 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























