I. What are Enzyme Kinetics?
When using the phrase “living life in the fast lane”, enzymes take it to a whole other level. Their basic core function is to speed up reactions to the best of their ability! We can honestly credit enzymes for the physiological functioning of our bodies, as their function builds the basis for the functioning of all the other crucial organs in our body.
Enzyme kinetics refers to the rate (or how fast) an enzyme catalyzes reactions! You’ll see while reviewing this article that how fast an enzyme catalyzes a reaction depends so much more than the enzyme itself: you’ll have to take into account how much enzyme is present to begin with, how much substrate it present, the affinity for the enzyme for a certain substrate, etc.
Aside from also having overlaps with chemical kinetics, understanding this topic lays the basis for understanding enzyme inhibitors and how they function (most likely a topic that’ll come up in your near future)! Let’s go ahead and get started on the basics now so that when you encounter enzyme inhibitors, you’ll be well prepared!
II. Content Review
It may help to first identify and define the main players involved in enzyme kinetics before getting into all the equations involved.
The binding interaction of the enzyme & substrate as well as product formation can be modeled via the following formula below:
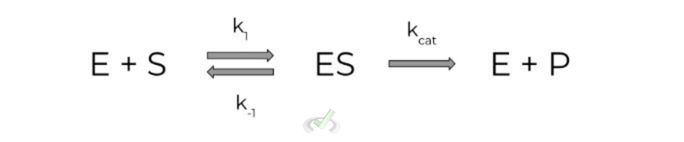
This basically states that the enzyme and substrate can form the enzyme-substrate complex at a rate k1 (and dissociate back to the free enzyme and substrate at a rate k-1).
In addition, the equation also states that the enzyme-substrate complex can form the product and free enzyme at a rate kcat (the “-cat” subscript comes from the fact that this is the reaction that needs to be catalyzed in order to form the products).
When talking about Michaelis-Menten equations for enzyme kinetics, we really only focus on kcat, as it will be the rate utilized in a majority of the equations.
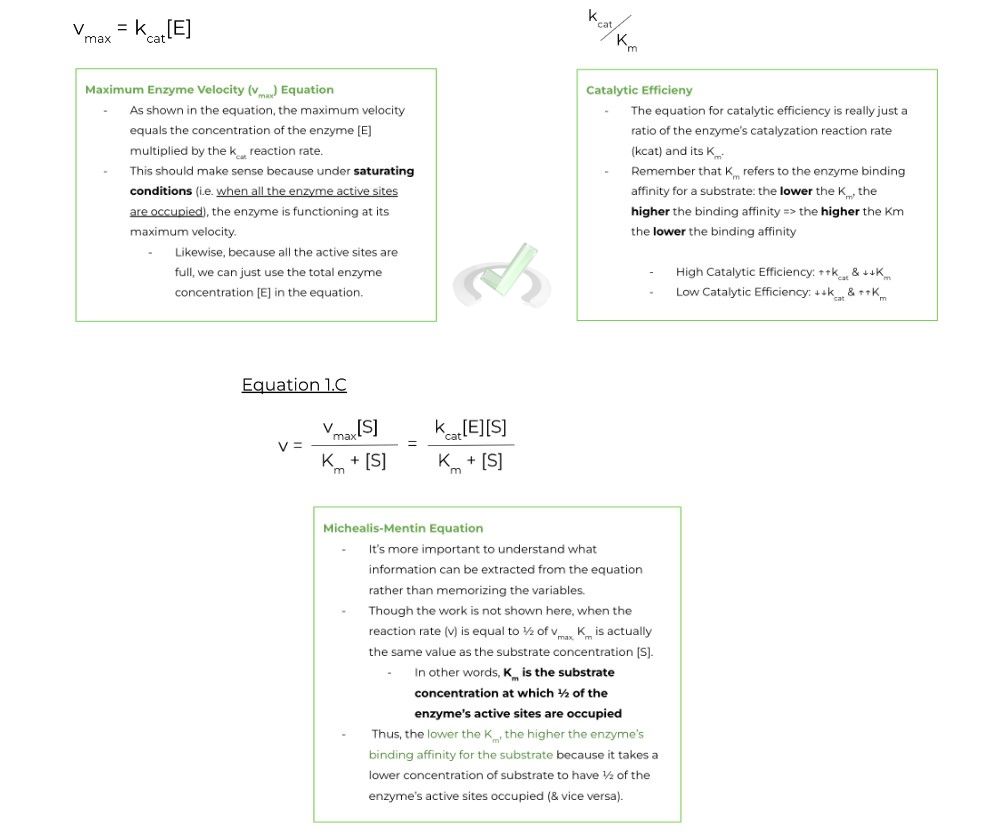
There are 3 main Michaelis-Menten equations (and 1 derivative) that you should be familiar with come test day.
You’ll most likely not be required to solve calculations when given this equation.
Rather, it’s more important to understand the relationship between the variables and how they connect back to understanding enzyme kinetics as a whole. We’ll talk about the following equations:
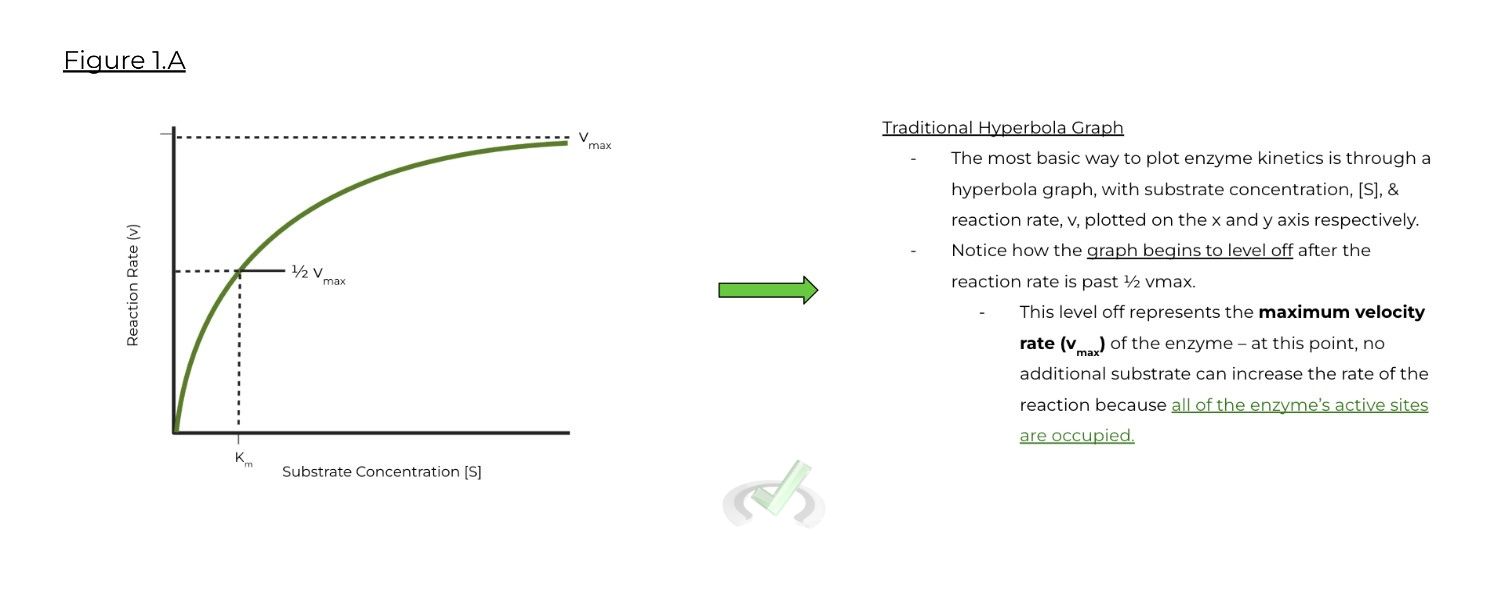
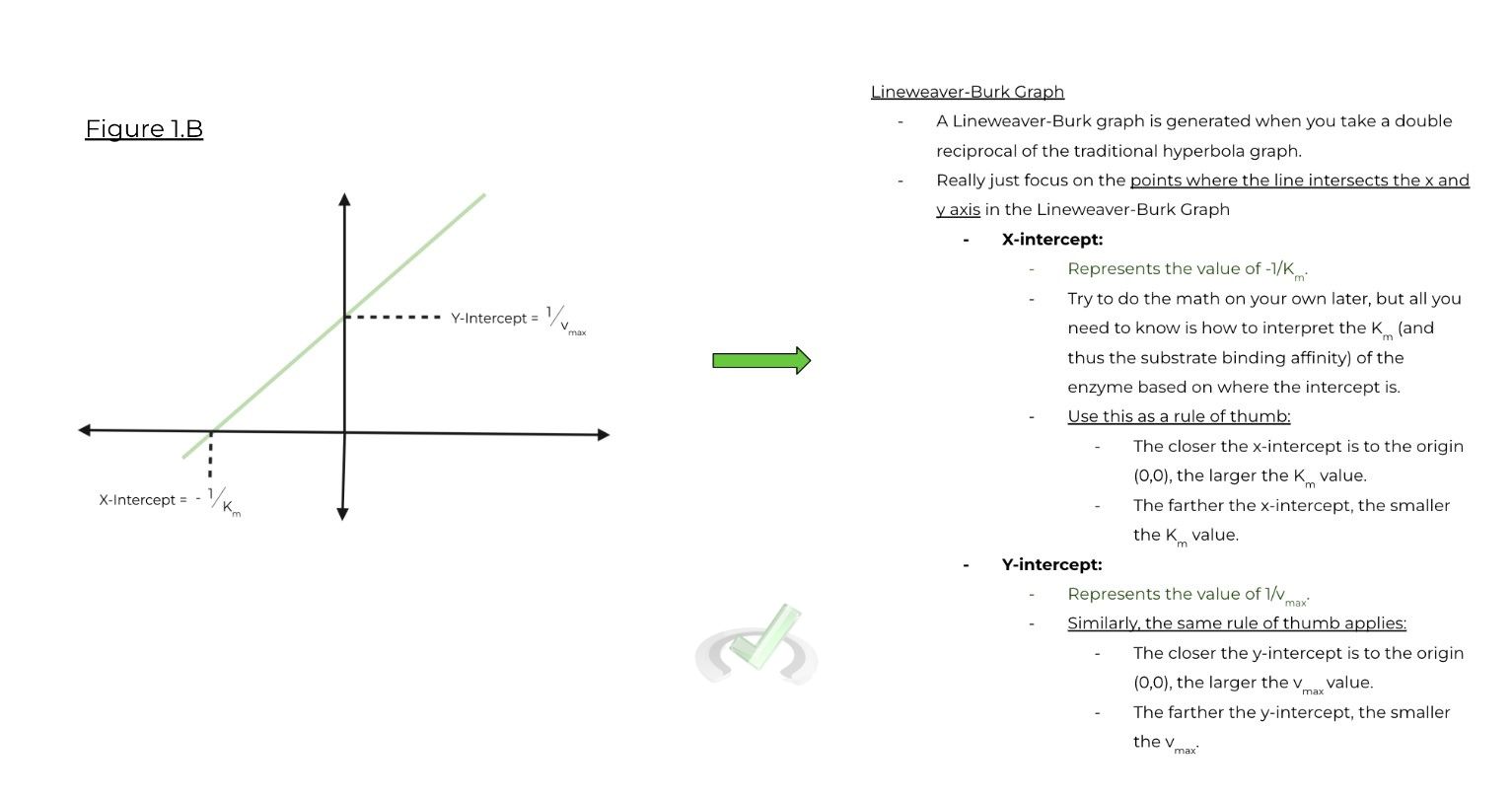
Graphs are additionally important (maybe even more important than the equations!) when understanding & analyzing enzyme kinetics. There are 3 types of graphs to be familiar with when discussion enzyme kinetics:
III. Bridge/Overlap
Probably the most applicable overlap in terms of content and application is through enzyme inhibitors! We’ll cover this in much more detail in the next section, but it definitely helps to get a good grip on the basics!
As such, the most important concept to have done in this section is the Lineweaver-Burk plots, as you’ll often see enzyme function plotted with & without the presence of a inhibitor.
Enzyme inhibitors will affect the Km and/or the vmax of the enzyme. Oftentimes when the MCAT tests this concept of enzyme inhibitors by asking you to classify a drug based on the type of inhibition.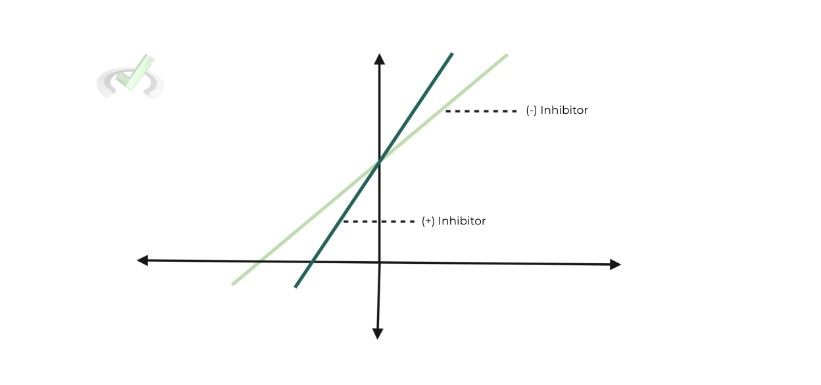
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
Enzyme Kinetics:
Refers to how fast an enzyme can catalyze a reaction!
There are 3 main equations (& 1 derivative) that you should be familiar with regarding enzyme kinetics. Don’t be too worried about being able to calculate the equations! We’ll leave you notes on what to focus on for each equation:
A. Maximum Enzyme Velocity (vmax) Equation:
a. When all the enzyme’s active sites are full, the enzyme is fully saturated and functioning at its maximum velocity. Hence, we can use the total concentration of enzyme, [E], multiplied by the kcat to get the maximum velocity, vmax.
B. Catalytic Efficiency:
a. Acts as a ratio between the enzyme’s kcat and Km
i. A high catalytic efficiency = ↑↑ kcat, ↓↓ Km
ii. A low catalytic efficiency = ↓↓ kcat, ↑↑ Km
C. Michaelis-Menten Equation:
a. When the reaction rate (v) is ½ of vmax, the substrate concentration, [S], is equal to Km. In other words, Km is equal to the substrate concentration at which half of the enzyme’s active site is occupied.
i. The lower the Km, the higher the binding affinity because it takes a lower substrate concentration to have ½ of the enzyme’s active site filled (& vice versa).
Graphs are also an important concept to become familiar with when studying enzyme kinetics. Similar to the enzyme kinetic equation, we’ll leave you notes on what to focus on!
A. Traditional Hyperbolic Graph:
a. Usually the most basic way to display enzyme kinetics. The leveling off of the curve represents when the enzyme approaches maximum velocity, as no additional substrate will increase the reaction rate.
B. Lineweaver-Burk Graph:
a. Really just pay attention to the x and y intercepts in order to determine the values of Km & vmax.
i. X-intercept: Represents value of -1/Km
ii. Y-intercept: Represents value of vmax
1. A simple rule of thumb:
a. The closer the intercept is to the origin (0,0), the larger the value of Km/vmax is (& vice versa).
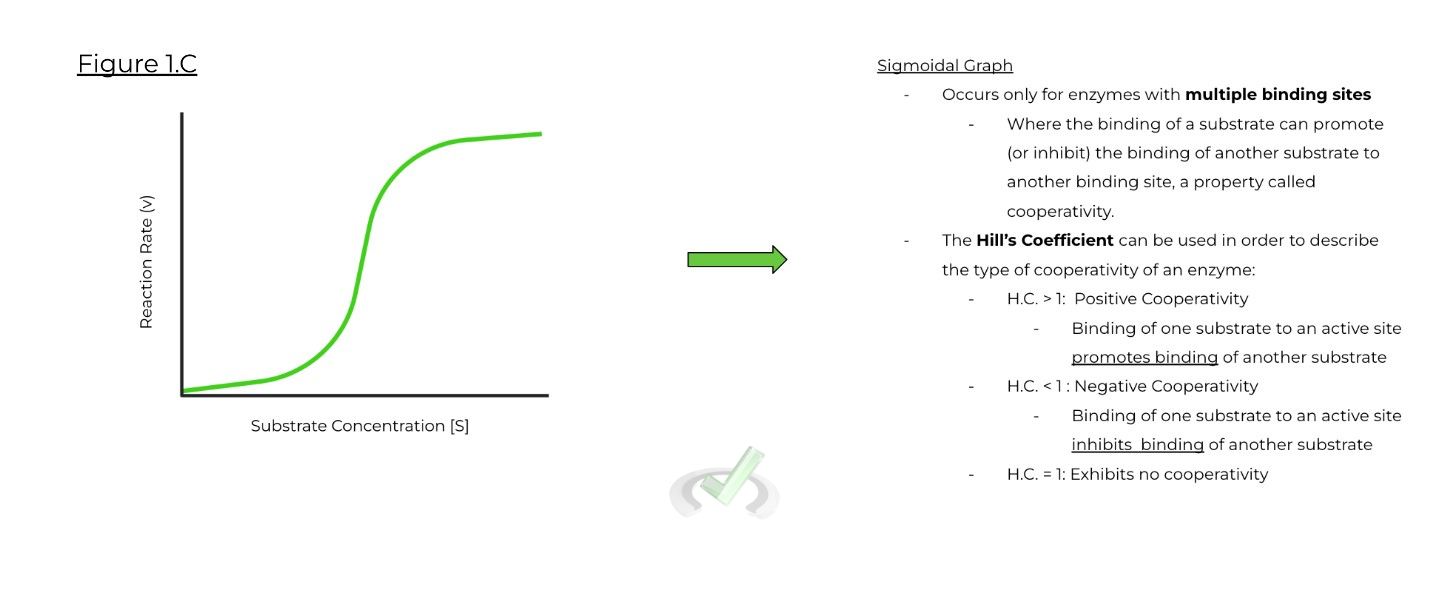
C. Sigmoidal Graph:
a. Only occurs for enzyme’s with multiple binding sites! => The binding of one substrate to one binding site can either promote or inhibit the binding of another substrate to another binding site (called positive or negative cooperativity, respectively).
b. Hill’s Coefficient is a number that can describe the cooperativity of an enzyme:
i. H.C. > 1: Positive Cooperativity
ii. H.C. < 1: Negative Cooperativity
iii. H.C. = 1: No Cooperativity Exhibited
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
The Lineweaver-Burk graph shown below displays the enzyme kinetics of 2 enzymes (which have the same substrate): A and B. Which of the following statements best describes the difference in enzyme kinetics between the 2 enzymes?
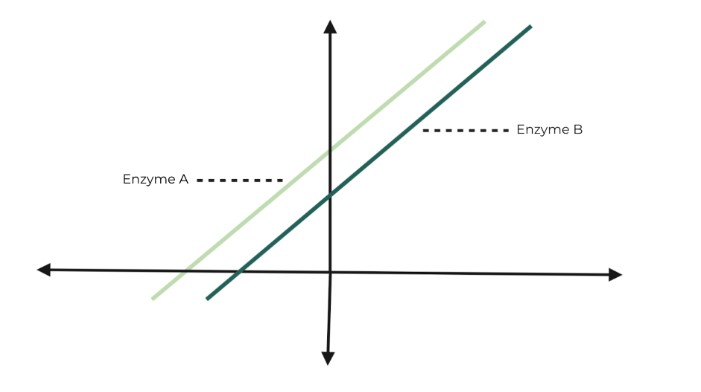
A. Enzyme B will have a higher binding affinity for the substrate.
B. Enzyme A has a lower maximum reaction rate.
C. Enzyme B has both a lower Km and a maximum reaction rate.
D. Enzyme A has both a higher Km and a maximum reaction rate.
Ans. B
Really remember the rule of thumb that we discussed in the article to solve this problem: the closer the intercept is to the origin, the higher the value of both the Km and vmax. Because enzyme A’s y-intercept is farther away from the origin, it will have a LOWER vmax than enzyme B.
Enzyme A will have the higher binding affinity for the substrate because it has the lower Km value!
Enzyme B will both have a HIGHER Km & vmax value because its x and y intercepts are closer to the origin (meaning they will have a higher value). .
Enzyme A will both have a LOWER Km & vmax value because its x and y intercepts are farther from the origin (meaning they will have a lower value).
Sample Practice Question 2
The chart below gives a list of the Km & kcat values for a wildtype enzyme and various mutant forms of the enzyme after single amino acid substitutions. Which of the following will cause the most negative change to the enzyme’s catalytic efficiency?
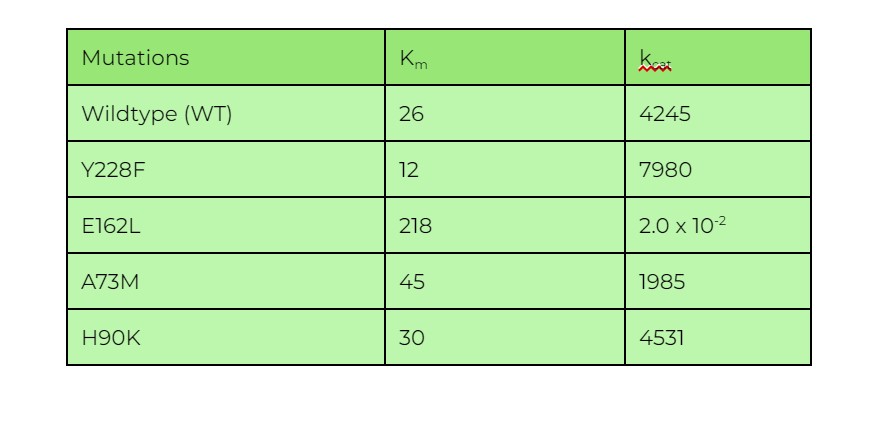
A. Y228F
B. E162L
C. A73M
D. H90K
Ans. B
Catalytic efficiency is essentially a ratio between the enzyme’s kcat and its Km, with a higher value meaning that the enzyme is more efficient in catalyzing a reaction.
In order to find the mutation which causes the most NEGATIVE effect on catalytic efficiency, we have to find the mutation which:
Out of all the mutations, it’s evident that the E162L has the most drastic increase in the enzyme’s Km (indicating low binding affinity) and the most drastic decrease in kcat (which means a much lower vmax). As such, this E162L mutation will result in the most negative effect in the enzyme catalytic efficiency.
VI. Additional Links
Here are some additional links to related articles & additional resources that will hopefully be helpful to you all!
“Enzyme Models & Classification” Study Article: https://docs.google.com/document/d/19M0DOTtCa-MkhItt4utgG-HHr8n1xnxsy_I_p9cWBq8/edit?usp=sharing
“Enzyme Structure & Function” Study Article:
https://docs.google.com/document/d/1knFYiDcT_vZ1lZlJHlCQOuZbjTxXjYTQYwRkd-Cvr1I/edit?usp=sharing
“Enzyme Kinetics & Inhibitors” Quicksheets:
TBA



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























