I. What are the Non-Enzymatic Functions of Proteins?
The beauty of biochemistry stems from the wide variety of microcellular molecules and substances that make our body function in tip top shape! If you need one example, look no further than the vast collection of proteins and their functions!
While enzymes are probably the first thing that comes to mind in terms of protein function, there are numerous other proteins with differing functions which all work together in an interconnected system in order to perform basic and advanced cellular processes!
Though this article goes in detail about non-enzymatic proteins, always look to make comparisons and analogies between these proteins and enzymes! Still keep in mind amino acid placement, basic protein structural levels, and all the other basics of protein structure!
II. Different Types of Non-Enzymatic Proteins
We’ll primarily cover 3 main types of non-enzymatic proteins: 1) binding, 2) immune, and 3) motor proteins. Being non-enzymatic, these proteins fulfill roles such as structural integrity movement, etc.
A. Binding Proteins
As indicated by their names, these proteins are involved with the binding of various substances. These can be for a variety of reasons from transporting molecules to signal transduction as seen in a receptor.
Binding proteins contain a binding domain which binds to a molecule of interest due to the formation of favorable interactions between the domain and molecule.
To give a few examples, let’s look at hemoglobin and DNA binding transcription factors and see their functions as binding proteins!
I. Hemoglobin
You may already be familiar with this protein! Hemoglobin actually has 4˚structure being composed of 4 subunits, each with a heme group containing a central iron atom.
The heme group and specifically the central iron atom allow for the oxygen binding through forming a coordinate covalent bond interaction.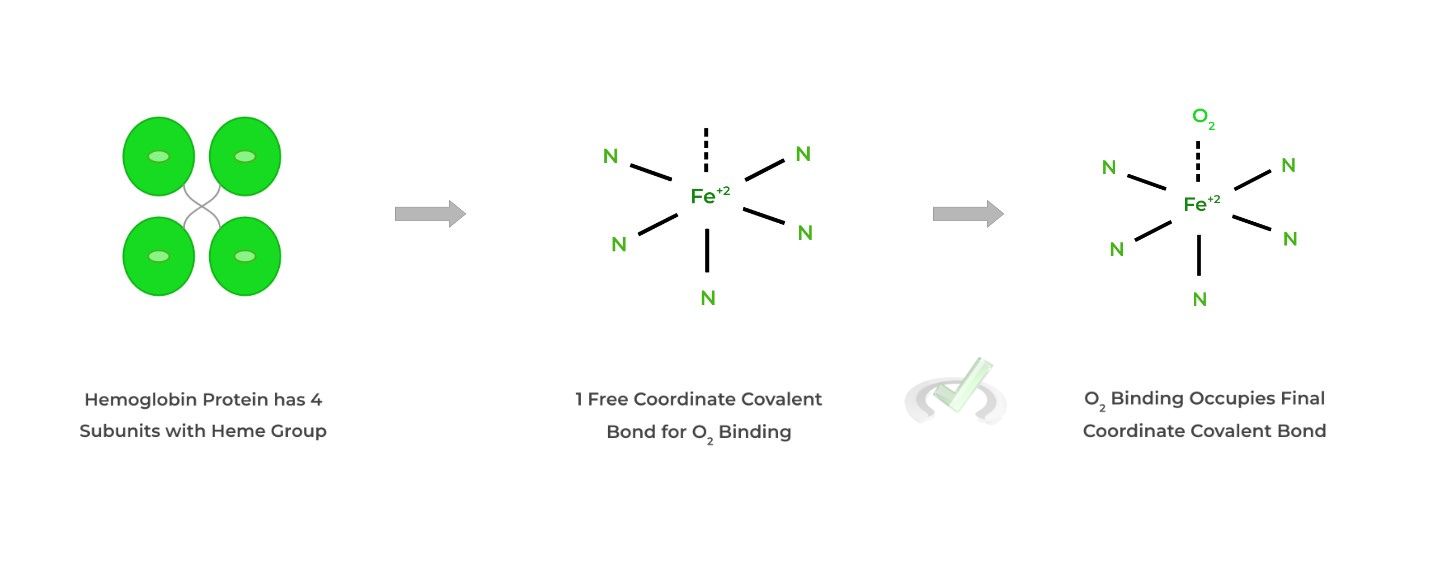
Hemoglobin is responsible for the binding and transportation of oxygen to tissues. It’s also unique in the sense that it participates in cooperative binding, where the binding of O2 to one subunit increases the likelihood of O2 binding in another subunit!
II. DNA Binding Transcription Factors
As indicated by its name, these transcription factors bind to the DNA, specifically at the phosphate backbone, in order to promote or inhibit transcription.
Because the phosphate backbone is the primary binding point for these proteins, their DNA binding domains will generally have basic amino acids!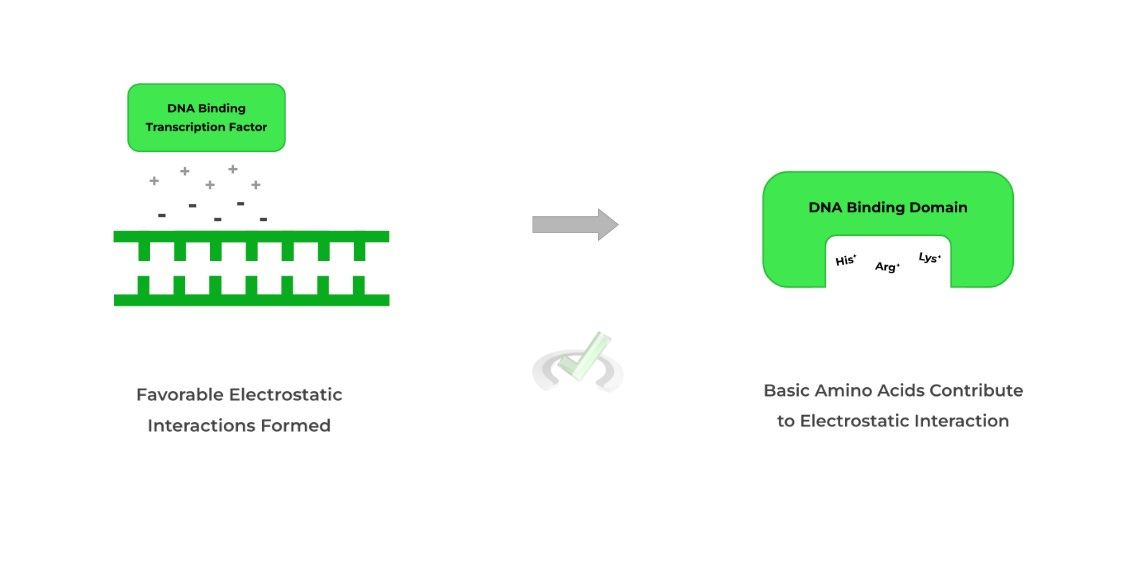
This is due to the favorable electrostatic interactions between the positively charged amino acids and the negatively charged phosphate backbone.
B. Immune Proteins
As their name suggests, these proteins are heavily involved in the functioning of the immune system! Let’s discuss one of the major ones: antibodies.
I. Antibodies
These immune proteins are Y-shaped soluble proteins produced by B cells which can bind to pathogens and prevent their infection.
Like hemoglobin, they also have 4˚ structure, being composed of a light and heavy chain. The light and heavy chain together make 2 different regions: 1) variable, antigen binding region, and the 2) constant region.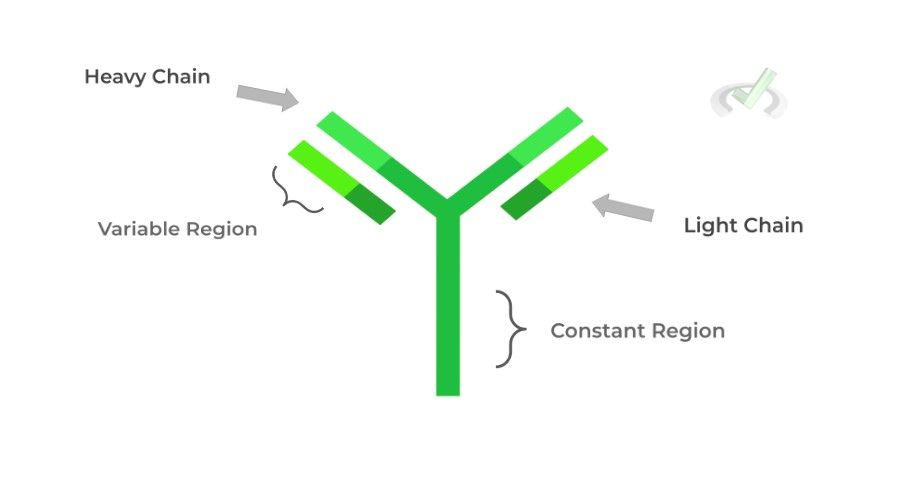
The binding of antibodies to pathogens can help neutralize in many ways, as covered in more detail in our immune system articles!
C. Motor Proteins
Likewise, these proteins have motor function and are involved in movement! Again while there are many to cover, we’ll focus on 1) myosin and 2) kinesins and dyneins.
I. Myosin
You may also be familiar with this protein, as it’s involved in the contraction of muscles! Though covered in more detail in our musculoskeletal articles, we’ll offer a brief overview!
Simply put, myosin proteins interact with the thin, actin filaments and “pulling” them closer to allow for sarcomere shortening, resulting in muscle contraction!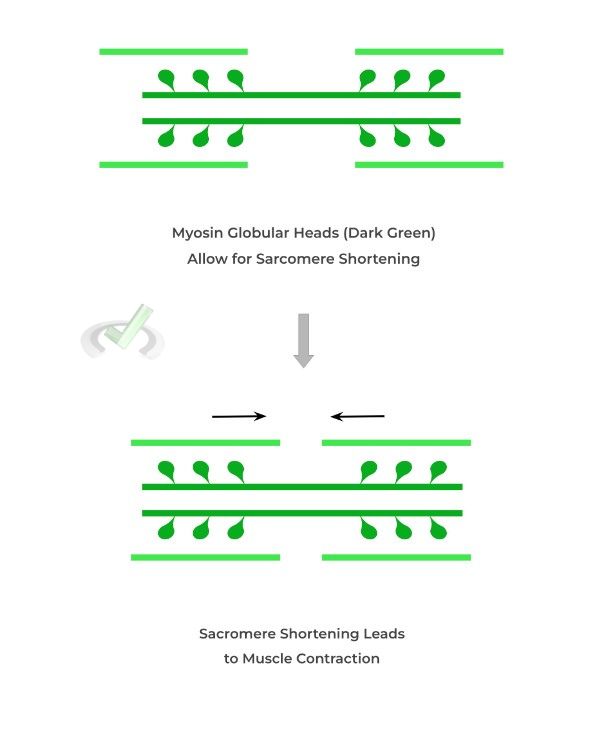
II. Kinesins and Dyneins
These proteins are primarily involved in vesicular transport, often utilizing microtubules as transport paths within a cell, similar to a car on the freeway! The main difference between kinesins and dyneins are in their direction!
Kinesins participate in anterograde transport, where movement is toward the cell exterior. Dyneins take part in retrograde transport, moving towards the cell interior.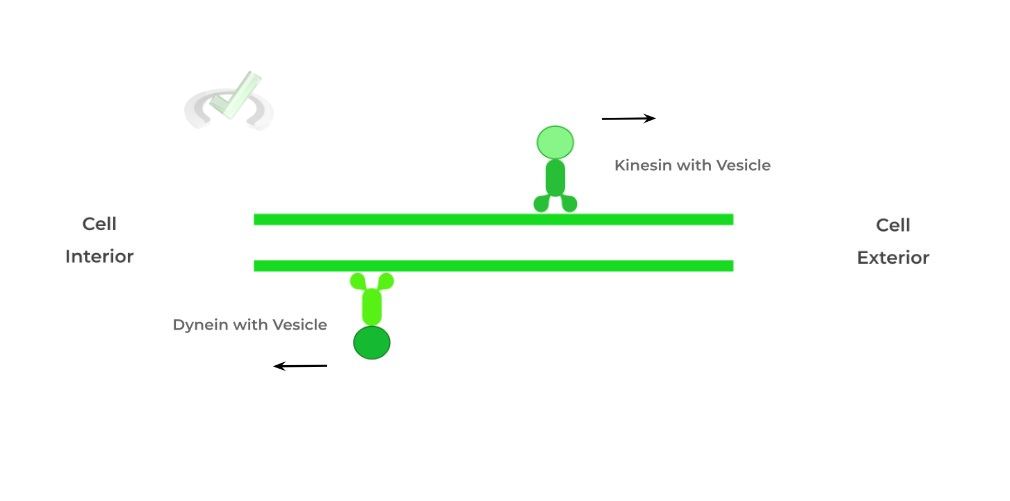
III. Bridge/Overlap
Just like we always encourage here at MCAT Mastery, we’ll give you a real, cellular example of the proteins above! We’ll give you all an example of kinesin and dynein functioning as we’ve already given some examples of the other proteins!
I. Neurotransmitter Transport via Kinesins and Dyneins
Recall that neurons have a cell body (soma), axon, and synaptic boutons (axon terminals). The neurotransmitters, which are stored within the vesicle in the synaptic boutons waiting for release, are first produced in the soma as this is where all the cell machinery is located!
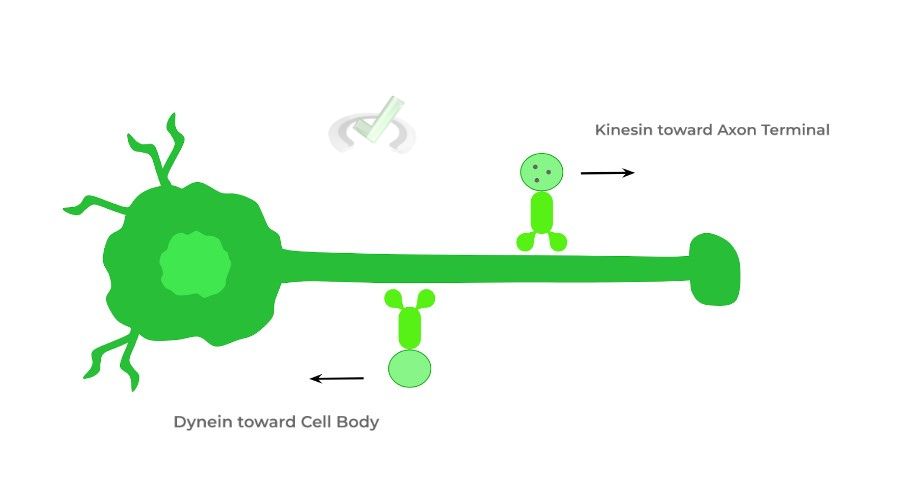
Here, kinesins utilize microtubules along the axon to travel towards the axon terminals to deliver the neurotransmitters.
When the neurotransmitters are released and vesicles are empty, dyneins can return the vesicles to the soma to be packaged again with neurotransmitters.IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Binding Proteins
As implied by their names, these proteins are involved with the binding of various substances to be used from transporting molecules to signal transduction.
Each binding protein contains a binding domain, which forms favorable interactions with its molecule of interest.
I. Hemoglobin
Involved in the circulatory system, hemoglobin is responsible for the binding of oxygen molecules and transports them to tissues.
The protein contains 4 subunits, each with a heme group and central iron atom. The iron atom forms a coordinate covalent bond with oxygen which allows for binding.II. DNA Binding Transcription Factors
These proteins bind to the phosphate backbone of DNA in order to regulate transcription! Because they target the phosphate backbone, the DNA binding domains generally have basic amino acids!
The positively charged amino acids form favorable electrostatic interactions with the negatively charged phosphate backbone.B. Immune Proteins
The most important protein involved in the immune system are antibodies, which are Y-shaped soluble proteins produced by B cells.
Antibodies are composed of a light and heavy chain. Together the light and heavy chain form 2 different regions: 1) variable, antigen binding region and the 2) constant region.
The binding of antibodies to pathogens allows for their neutralization in many different ways, as explained more in our immune system articles.
C. Motor Proteins
As suggested by their names, these proteins have motor function and are involved in movement, including myosin and kinesins/dyneins.
I. Myosin
This protein is most heavily involved in muscle contraction, where it interacts with thin, actin filaments to allow for sarcomere shortening.
II. Kinesins/Dyneins
These proteins are primarily involved in vesicular transport, associating with microtubules and using them as cellular transport pathways!
Kinesins participate in anterograde transport for movement towards the cell exterior while dyneins participate in retrograde transport for movement towards the cell interior.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
The AR protein is responsible for binding testosterone and initiating a signal cascade cia binding through the ligand binding domain. What amino acids are expected to be present in the ligand binding domain?
A. F, L
B. C, S
C. D, E
D. H, R
Ans. A
Remember that testosterone is a nonpolar, hydrophobic molecule! Thus, nonpolar amino acids such as phenylalanine and leucine should be present in the ligand binding domain for favorable interactions.
Sample Practice Question 2
Suppose THK is an inhibitor of a DNA binding transcription factor. THK will use its inhibitory domain to bind to the DNA binding domain and sequestering the transcription factor. Which amino acids do you expect to be present on the inhibitory domain of THK?
A. F,L
B. C, S
C. D, E
D. H, R
Ans. C
Take this question one step at a time! Remember that the DNA binding domain of the transcription factor will generally have positively charged amino acids to form the electrostatic interaction with the negatively charged phosphate backbone.
n order to bind to the DNA binding domain and sequester the transcription factor, the inhibitory domain should also have negatively charged amino acids to form favorable interactions with the positively charged amino acids on the DNA binding domain.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























