I. What is Adenosine Triphosphate (ATP)?
When talking about what cell’s use for energy, this molecule takes the top spot! If you’ve taken a biochemistry class or even introductory biology lectures, you’ll most likely have heard of this molecule and its importance to various cellular processes.
It’s without a doubt you’ll come across this molecule numerous times during your MCAT prep and MCAT content review, but it is for good reason! As we’ll discuss in detail in a bit, this molecule helps power many of the cellular reactions that keep our body functioning.
When going through this article, think about all the different reactions that utilize ATP, helping to appreciate the importance of this molecule. As such, we’ll cover this topic as concisely and succinctly as possible, making sure you get only the necessary information that you’ll need to ace your exam!
II. The Importance of Adenosine Triphosphate
It’s first best to review the structure of ATP before getting into its function as an energy source and the mechanisms behind its function!
A. ATP Structure
Luckily, the name of the molecule already outlines a little bit of its structure: an adenine base is connected to a ribose sugar on the C1 carbon as well as 3 phosphate groups, connected by phosphoanhydride bonds.
The phosphate groups are labeled as alpha (𝜶), beta (𝜷), and gamma (𝜸) from the nearest to ribose sugar to the most terminal, respectively.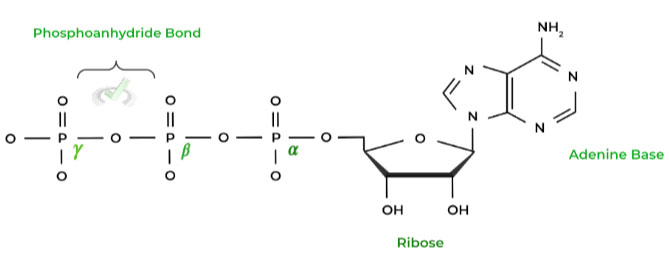
It’s important to note that ATP is a high energy molecule, due to the negatively charged phosphate groups, as their proximity causes electrostatic repulsion, which contributes to the energy release when a phosphate group is released as we’ll discuss further below!
B. Reactions of ATP
When one (or more) phosphate(s) group is removed from ATP, it’s accompanied by a fair release of energy as mentioned above. There are 2 main reactions that are associated with ATP: 1) ATP hydrolysis and 2) phosphoryl group transfer.
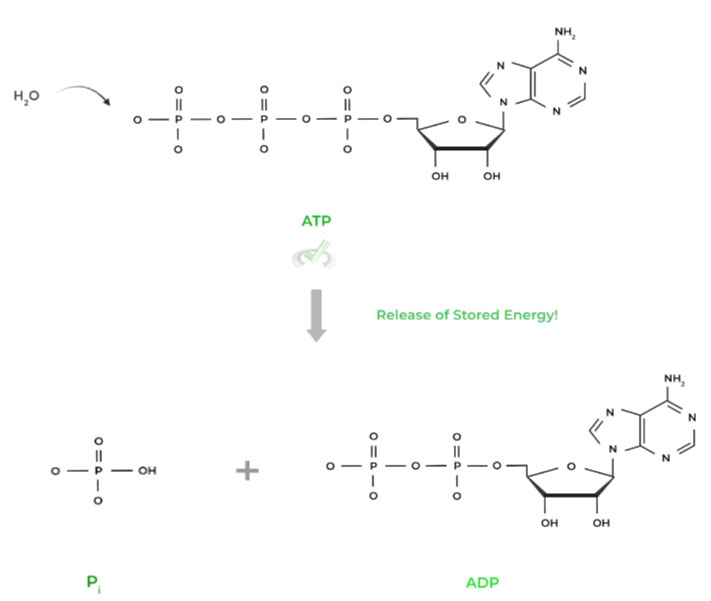
I. ATP Hydrolysis
As with all hydrolysis reactions, a water molecule acts as a nucleophile which substitutes the gamma phosphate group, releasing it. Accompanying this is a big release of free energy, specifically around -30.5 kJ/mol under standard conditions.
II. Phosphoryl Group Transfer
This is in a way similar to the above reaction, except that instead of generating a free phosphate, the group is instead transferred to another molecule.
In this case, instead of a water molecule, another group, usually an -OH group, will act as the nucleophile and attack the gamma phosphate. Look at the example below!
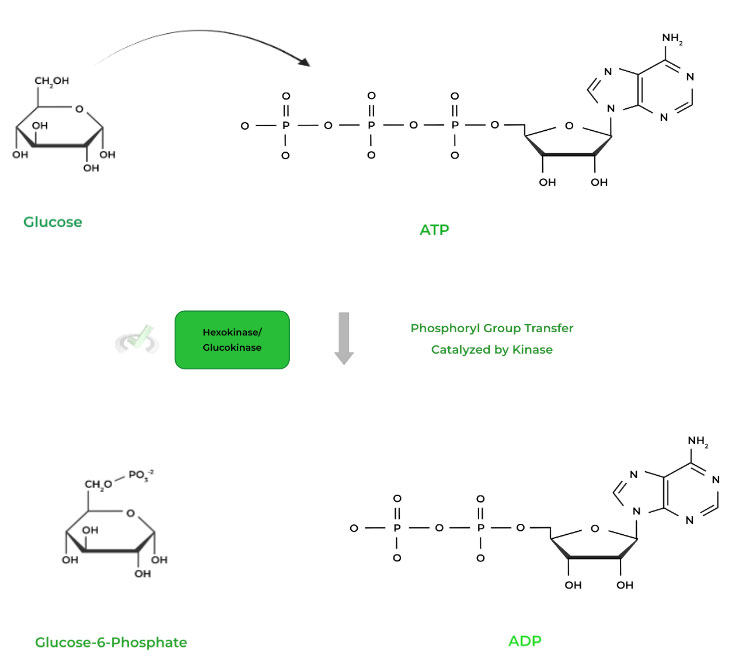
As mentioned above, the kinase catalyzes the transfer by catalyzing the nucleophilic substitution of the -OH group to the ATP molecule.
Oftentimes, the phosphorylation that results from this phosphoryl group transfer results in the phosphorylated molecule being more thermodynamically favorable for subsequent reactions!
III. Bridge/Overlap
Because ATP hydrolysis releases a great amount of energy, we can actually use that energy to power thermodynamically unfavorable reactions (i.e. nonspontaneous).
This process is called coupling, as we’re pairing 2 reactions together, with one reaction providing the free energy to drive the other, which is best shown through Hess’ Law.I. Coupling and Hess’ Law
Let’s look at a real example to better understand this concept. The synthesis of sucrose from glucose and sucrose is highly thermodynamically unfavorable, as indicated by the large, positive 𝜟G˚of +27 kJ/mol. However, we can couple this to ATP hydrolysis!
Recall that ATP hydrolysis is thermodynamically favorable because of the instability that results from the electrostatic repulsion in the phosphoanhydride bonds.
Its hydrolysis results in a release of energy which can power the synthesis of sucrose from glucose and fructose!
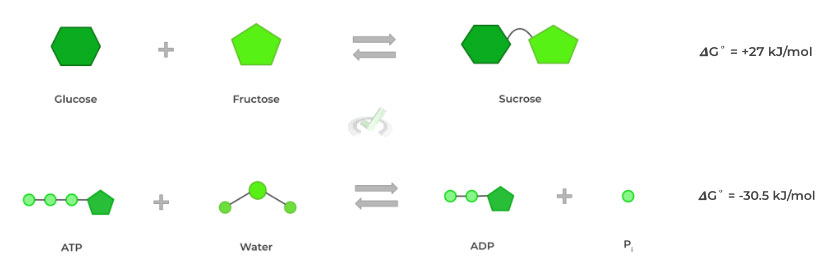
We can then use Hess’ Law to find that the change in Gibbs free energy for the coupled reaction is 𝜟G˚= -3.5 kJ/mol, now making the synthesis of sucrose from glucose and fructose thermodynamically favorable!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. ATP Structure
The name itself luckily contains some of the information about its structure! ATP is composed of a ribose sugar with an adenine base attached at the C1 carbon and 3 phosphate groups.
The phosphate groups are all linked by phosphoanhydride bonds and are termed alpha, beta, and gamma, from the one closest to the sugar to the most terminal, respectively. .
The close proximity of the negatively charged groups causes electrostatic repulsion, which has a fair amount of stored energy which can be released!B. Reactions of ATP
As mentioned above, the release of a phosphate group from ATP is accompanied by a fair release of energy, which is seen in ATP hydrolysis and phosphoryl group transfers.
I. ATP Hydrolysis
Similar to other hydrolysis reactions, an incoming water molecule acts as a nucleophile which substitutes the terminal, gamma phosphate group.
The hydrolysis is accompanied with a release of energy, specifically around -30.5 kJ/mol under standard conditions, which can be coupled to power other thermodynamically unfavorable reactions.II. Phosphoryl Group Transfer
In this case, instead of a free phosphate group being generated, the group is instead transferred to another molecule, which contains another nucleophile, usually an -OH group,
Oftentimes, the transfer of a phosphoryl group is done in order to make a molecule or substrate more thermodynamically favorable for subsequent reactions!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Which of the following is the best term to describe ATP and why?
A. Nucleotide; Because it contains a nitrogenous base, a sugar, and 1 (or more) phosphate group(s)
B. Nucleotide; Because it contains a nitrogenous base and a sugar
C. Nucleoside; Because it contains a nitrogenous base, a sugar, and 1 (or more) phosphate group(s)
D. Nucleoside; Because it contains a nitrogenous base and a sugar
Ans. A
ATP has all the components (nitrogenous base, sugar, and 1 or more phosphate groups) to be termed a nucleotide. Remember to understand the difference between the 2 terms!
Sample Practice Question 2
Which of the following combinations is the best way to describe ATP hydrolysis?
A. Exergonic; Spontaneous
B. Exergonic; Nonspontaneous
C. Endergonic; Spontaneous
D. Endergonic; Nonspontaneous
Ans. C
When looking at the structures of aspartate & lysine, it can be seen that aspartate does have an extra carboxyl group while lysine does have an extra lysine group.
This should make sense, as in physiological pH, the carboxyl group is deprotonated and takes on a negative charge (-1) while the amino group is still protonated and takes on a positive charge (+1).
Hence, this is why aspartate is a negatively charged amino acid while lysine is a positively charged amino acid.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























