I. What is Gel Electrophoresis?
Besides sounding like something like a spell out of Harry Potter, you’ve probably done this lab technique if you’ve taken a basic biology lab class or even doing research! Nevertheless, this is a fundamental lab technique that’s utlilized in a wide variety of biological research!
In addition to being a widely utilized lab technique, it’s an essential component that’s tested heavily on the MCAT, as it employs a variety of overlap between other subjects such as general chemistry and biochemistry and requires you to synthesize these concepts to develop a strong understanding!
In addition to synthesizing different concepts and repeated exposure to practice problems, another way we believe that can aid in the learning in this topic is the use of visuals, which we’ll definitely employ as a tool in the article!
II. Gel Electrophoresis
Before we get into the main types of gel electrophoresis, like always it’s best to start with an overview and understand the basic principles that underlies the experimental technique.
A. Overview
Simply put, gel electrophoresis is a lab technique that can be used to separate and characterize mixtures of nucleic acids and proteins. Let’s break down the term itself to get a better understanding!
The “gel” component refers to the gel matrix (usually a polyacrylamide gel), which contains pores and branches which allows for the separation of the molecules via size!
The “electrophoresis” component refers to the electric field applied onto the gel matrix, where one side of the gel acts as the anode as it’s positively charged while the other side acts as the cathode as its negatively charged, separating molecule via charge!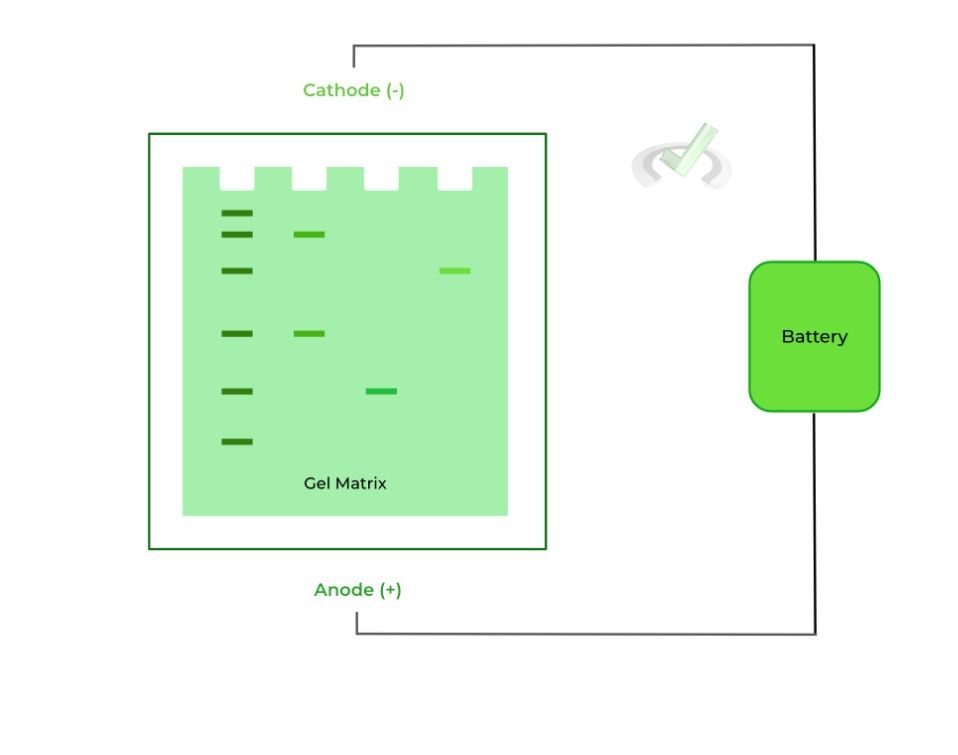
As mentioned above, the porous matrix gel and the applied electric field help to separate the molecules via their size, charge, and shape. Let’s take a look at how they do so and the relationships that can be established!
I. Size
When traveling through the gel, the larger molecule travels slower and a shorter distance towards the gel ends (depending on the charge). Conversely, the smaller molecule travels faster and a greater distance.
See if this analogy helps! Imagine a running back sees a hole in the defense to run through for the touchdown. Which would have an easier time running through the hole: a small, slim or a large, stocky running back?
Assuming they have the same strength, the smaller running back would run through the hole easier and faster because they’re just small enough to fit through the free space of the hole!
II. Charge
In this case, it’s relatively simple! The negatively charged molecules will migrate to the positively charged anode while the positively charged molecules will travel towards the negatively charged cathode via electrostatic interactions.
It’s important to remember the respective charges of the anode and cathode! One way to remember is that the cathode and anode follow the same charges as an electrolytic cell, as it also has a positive anode and negative cathode.
III. Shape
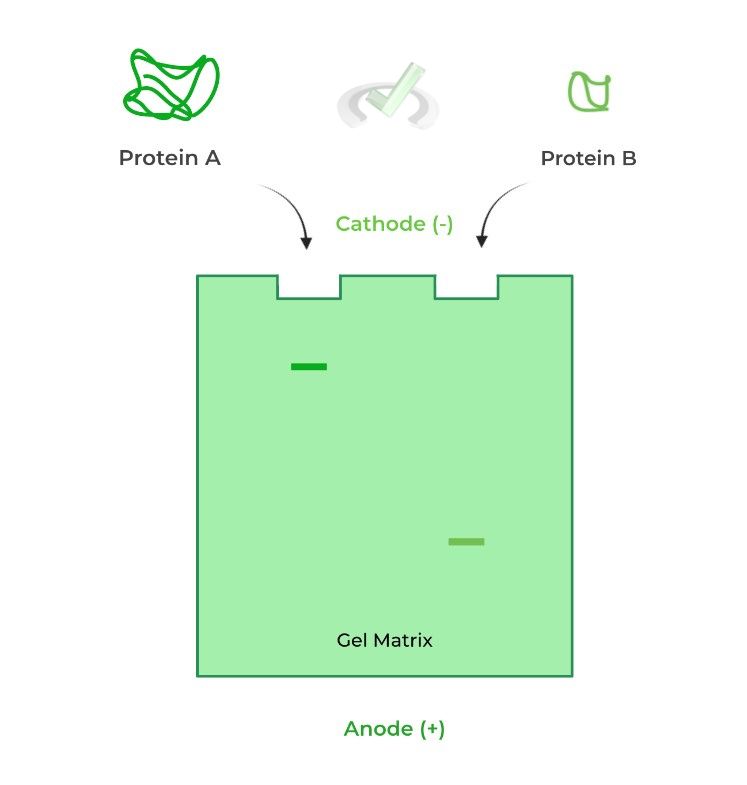
This factor requires you to think a lot more abstractly and more aptly applies to when studying proteins! In general, a more streamlined protein will also travel faster and at a greater distance while a more clumpy protein will travel slower and a shorter distance.
Picture this analogy to get a better understanding! When straining pasta noodles, which is more likely to pass through the strainer: a thin angel hair pasta or a clumpy lasagna pasta? – the angel hair!
Consider also the example below! We have 2 of the exact same proteins with the same amino acid length (20 kDa); however, one is in its tertiary 3D conformation while the other is in its primary structural level.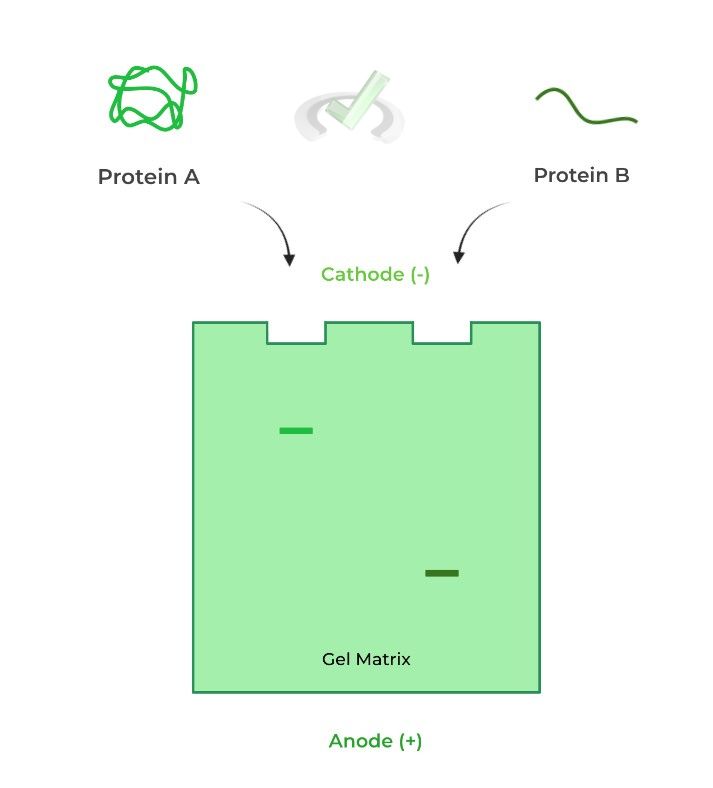
B. Types of Gel Electrophoresis
As mentioned, there are various types of gel electrophoresis that can be performed, which vary in terms of the conditions it’s performed under and what characteristic the variation is trying to exploit for separation (i.e. charge, size, etc.)
While gel electrophoresis can be done with nucleic acids, the following scenarios we’ll focus on are when proteins are being analyzed!I. Native PAGE
This one’s fairly easy to remember because researchers are focused on separating the proteins while still in their native form!
This includes all levels of structure, from 1˚ to 4˚, and the 3D protein conformation achieved from protein folding.
However, because the proteins are studied in their native form, it’s difficult to account for varying charges & protein shapes, providing a standard baseline. These limitations are usually why Native PAGE is not as widely tested compared to the other variations.II. SDS-PAGE
This technique is much more common to separate proteins, as here proteins can be separated on SIZE much more accurately!
Sodium dodecyl sulfate (SDS) is a detergent that’s applied to the proteins for 2 reasons: 1) It will disrupt any noncovalent interactions within the proteins and 2) It will create a uniform negative charge on the proteins.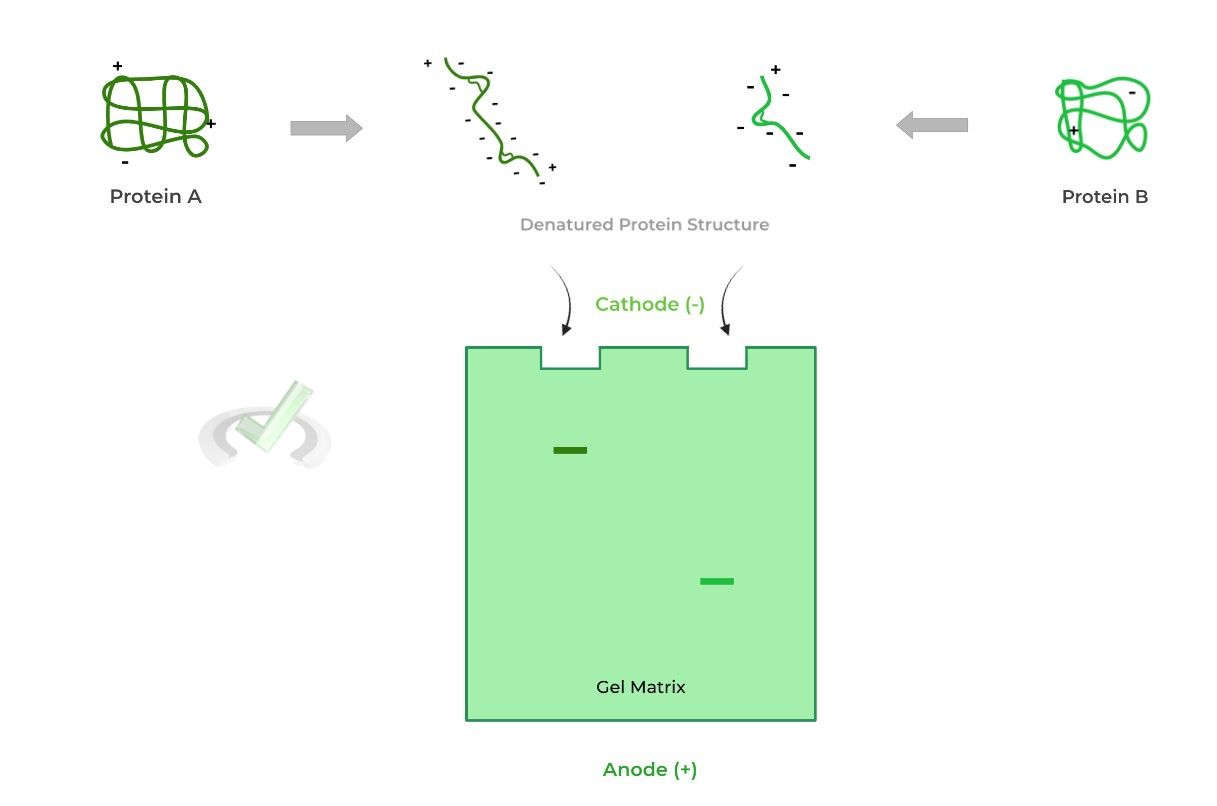
The disruption of the noncovalent interactions disrupts the 2˚, 3˚, and 4˚ protein structure (almost..). This denatures the protein from its 3D form and leaves a streamline, linear 1˚ structure, eliminating shape as a confounding variable.
Likewise, the addition of a uniform negative charge on the proteins eliminates charge as a confounding variable as now all the proteins will move towards the negatively charged cathode.III. Reducing SDS-PAGE
The one limitation to SDS-PAGE is that it cannot disrupt disulfide bonds, another component to 3˚ and 4˚ protein structure! You can kinda see this above where the proteins still have those disulfide connections, represented by the connected lines.
Reducing SDS-PAGE solves this by putting the proteins under “reducing conditions” which reduces the disulfide bonds back to the free thiol groups.
This is often used in order to see if there are subunits connected by disulfide bonds present within the 4˚ structure. One common reducing agent is beta-mercaptoethanol (BME)!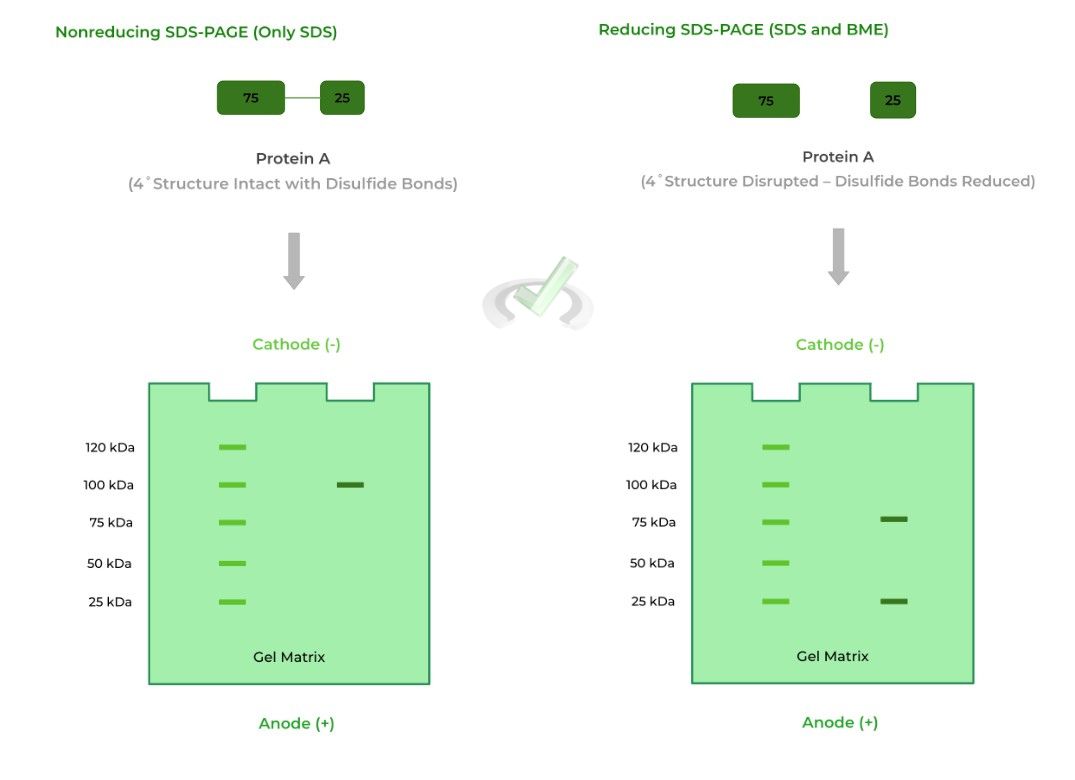
As shown above, protein A is actually a heterodimer, where the 4˚structure of the protein is composed of 2 different monomers: one 75 kDa monomer and one 25 kDa monomer.
In nonreducing conditions, only one band appears at the 100 kDa mark because the disulfide bonds connecting the monomers are not reduced. As such, it makes it seem that the protein is made up of only one subunit.
In reducing conditions, however, the disulfide bonds are reduced and broken which allows the monomers to separate. Now we see the monomers appear on the 75 and 25 kDa bands!
Note also that the left column of the gels is a reference point to determine the size of the protein in the experimental column.
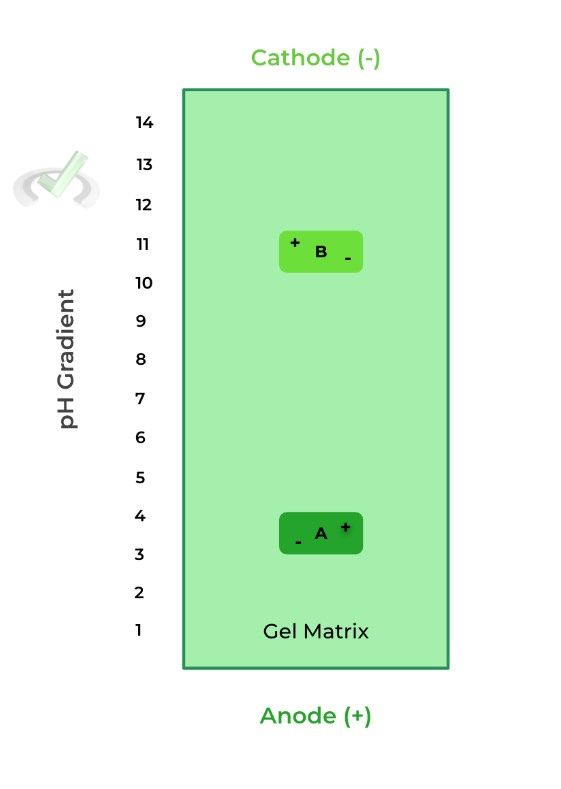
IV. Isoelectric Focusing
This type of gel electrophoresis takes advantage of the protein’s isoelectric point, which is the pH where the overall charge of the protein is neutral, similar to an amino acid’s pI!
Here, the gel is also embedded in a pH gradient, where the most acidic end (pH = 1) is located at the anode end and the most basic end (pH = 14) is located at the cathode end, as shown below.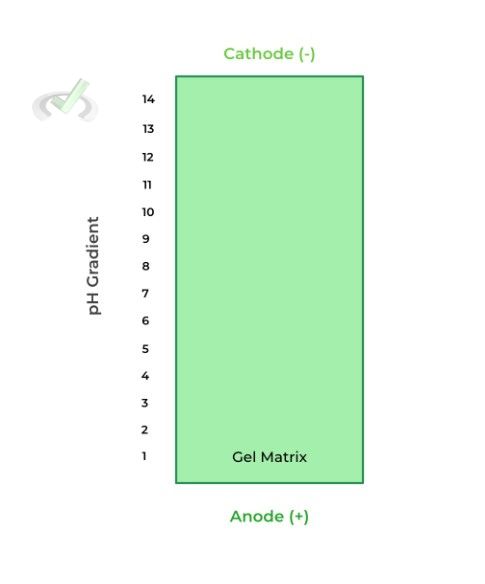
Isoelectric focusing takes advantage of the different protonation and charge states of acidic and basic amino acids. Recall that this is dependent on the pKas of the various groups and side chains!
Let’s look at an example of 2 different proteins: protein A as a pI of 3.5 while protein B has a pI of 10.8.
When the pH is less than the protein’s pI, it’s more positively charged because the increased H+ concentration results in more protonation, thus a more positive charge, moving it toward the cathode.
When the pH is greater than the protein’s pI, the protein is more negatively charged because the decreased H+ concentration results in less protonation, thus a more negative charge, moving it towards the anode.
Here, when protein A and B are placed in a pH of 7, they are negatively and positively charged, respectively. As such, protein A moves towards the anode and protein B moves towards the cathode, due to electrostatic interactions.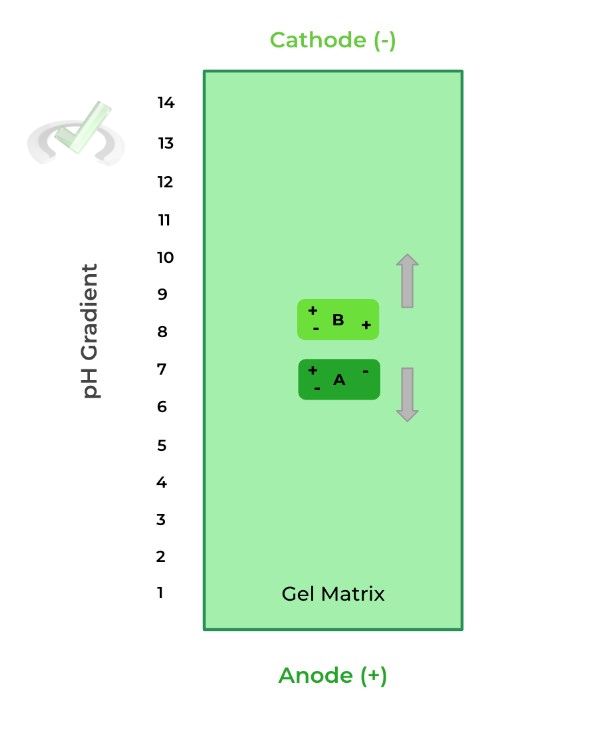
However, when protein A migrates towards the anode, the pH decreases which will make protein A gain positive charges; when protein B migrates towards the cathode the pH increases, making protein B more negative.
Eventually, protein A and B reach their isoelectric points where the gained positive and negative charges, respectively, results in an overall neutral charge of the protein.
At this point, the proteins do not further migrate because of lack of charge, allowing for separation.
III. Bridge/Overlap
As stated above, reducing SDS-PAGE conditions allows for the complete denaturation of the protein, allowing us sometimes to separate the monomer proteins present in 4˚ structures via the reduction of disulfide bonds. Need a quick refresher on disulfide bonds? We got you!
I. Disulfide Bonds & Oxidizing/Reducing Agents
Recall that disulfide bonds are formed via the oxidation of 2 sulfhydryl/thiol groups. These are often made by cysteine groups to stabilize 3˚ and 4˚protein structures, but can also be found in lipoates like those in dihydrolipoyl transacetylase!
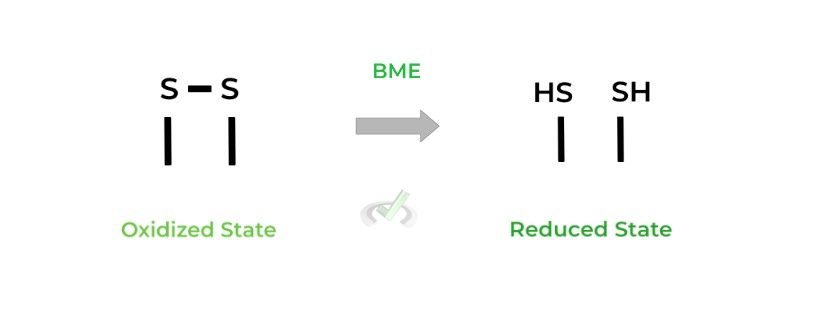
As stated above, these disulfide bonds are broken in reducing SDS-PAGE with the introduction of beta-mercaptoethanol (BME) which in this case acts as a reducing agent as it reduces the disulfide bonds back to free thiol groups.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Overview
Gel electrophoresis is a lab technique utilized to separate and study mixtures of nucleic acids and proteins.
The technique requires a gel-like, porous matrix and an applied electric field on the matrix, where one end is termed the anode (positively charged) and the other termed the cathode (negatively charged).
There are three main factors which determine how fast and what distance a molecule will travel in the gel: 1) size, 2) charge, and 3) shape.I. Size
A large molecule will travel a slower and a shorter distance while a small molecule will travel faster and a further distance.
II. Charge
Because of electrostatic interactions, negatively charged molecules will move toward the anode while positively charged molecules will move toward the cathode.
III. Shape
Similarly, a more streamlined and linear molecule (i.e. the more aerodynamic) will travel faster and a further distance than a clumpy, non-aerodynamic molecule.
B. Types of Gel Electrophoresis
There are various types of gel electrophoresis techniques which differ in the performed experimental conditions and the characteristic that is exploited for separation.
I. Native PAGE
Here, the protein is left in its native, 3D form and run on the gel. This is the most simple form of gel electrophoresis!
However, the limitations of this variation rely on the fact that there is no established uniform baseline (i.e. same charge/shape), which can create confounding variables.II. SDS-PAGE
In this case, a detergent called sodium dodecyl sulfate is used in order to 1) disrupt any noncovalent protein interactions and 2) establish a uniform negative charge on the protein.
This way, the proteins are denatured to a 1˚ structure (almost), which makes them both streamline, eliminating shape as a confounding variable.
Additionally, because the proteins are both negatively charged, they’ll both move towards the anode. Now, the proteins can be separated solely based on SIZE (i.e. the number of amino acids present!).III. Reducing SDS-PAGE
Think of this technique as a more specialized version of SDS-PAGE, where a reducing agent such as beta-mercaptoethanol is introduced to reduce disulfide bonds. This way the protein’s conformation is completely denatured.
This is very useful when discovering if there are disulfide bond connections within a protein's 4˚ structure!IV. Isoelectric Focusing
This variation takes advantage of a protein’s isoelectric point which is the pH at which the protein has a neutral charge! Here, the gel matrix also contains a pH gradient where the most basic end is at the cathode and the most acidic end is at the anode!
When the protein eventually reaches its pI, it will become neutrally charged and will stop moving; this allows for protein separation!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Given 2 DNA fragments, one 30 base pairs long and the other 80 base pairs long, which DNA fragment will travel farther and to which gel end?
A. 30 bp, Anode
B. 30 bp, Cathode
C. 80 bp, Anode
D. 80 bp, Cathode
Ans. A
In this case, both of the DNA fragments will travel to the same end, the anode, as both DNA fragments are negatively charged due to the phosphate backbone and will be electrostatically attracted to the positively charged anode end.
However the 30 bp will travel further as it's a smaller molecule: the smaller the molecule, the faster and further it will travel through the gel.
Sample Practice Question 2
Given the 2 gels below, what can be said about protein A? (Note: the left column on the gel is a protein size reference point).
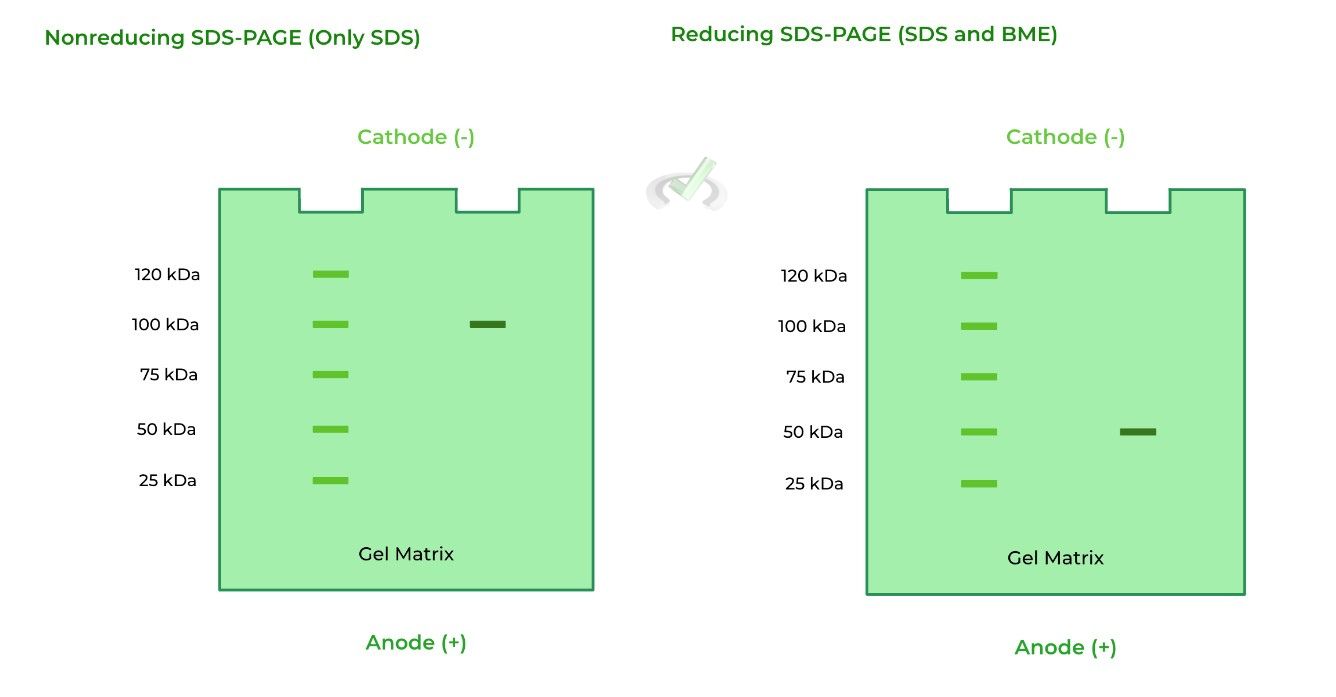
A. Protein A is a homodimer
B. Protein A is a heterodimer
C. Protein A is a homotrimer
D. Protein A has no 4˚ structure
Ans. A
Let’s take this one step by step! We see one band on the 100 kDa mark on the nonreducing SDS-PAGE. This may lead us to think that there is only one protein with no subunits, but it doesn’t account for disulfide bonds that are present in the 4˚ structures as this is nonreducing!
In reducing conditions, there is also only one band in the 50 kDa mark. However, we know that the protein should at least have 100 kDa of mass as shown in the nonreducing SDS-PAGE.
The best conclusion is that protein A is a homodimer, composed of two 50 kDa monomer subunits, which together equal 100 kDa.



 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
























