If you’ve already taken your general chemistry course in college to fulfill medical school requirements or maybe even already taken chemistry in high school, you may have heard your chemistry teacher say that chemistry is just like taking another math class.
While this claim does have its merits with all the calculations that the MCAT can test you on, this definitely the case for stoichiometric topics, as there are many numbers, conversions, and dimensional analysis that comes with this content.
Don’t worry if this scares you a little bit! We’ll give a brief introduction to the topics and calculations that are required for stoichiometry and will go into further detail in our in depth articles. Let’s hit it!
Stoichiometry on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try to expect around 2-4 questions that can appear on the MCAT covering stoichiometric topics, probably with a higher emphasis on calculation based questions!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Stoichiometry
Though stoichiometry is fairly math and calculation heavy, it doesn’t have to be daunting and scary! In fact, a lot of the calculations can be simplified if you’re careful that everything crosses out!
This “everything crosses out motif” is so important to understand because it will not only help you in getting to your answer but also in seeing if you made a mistake somewhere along your line of logic.
1. Quantification of Matter
Before getting into the calculations involved in stoichiometry — which is the general chemistry study of the quantitative relationships between substances in a chemical reaction — we must first get a grasp of the units we use for small molecules/particles.
There are 4 important values to go over when quantifying matter on such a small scale as in the case of atoms and molecules: atomic weight, molecular weight, moles, and molar mass. Let’s now go over these high yield values!
>> Atomic Weight:
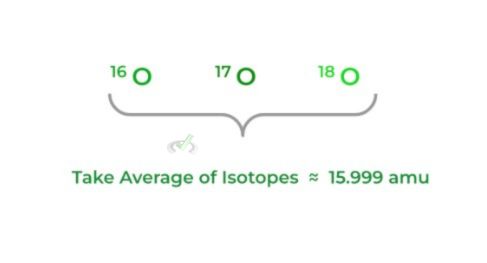
Defined as the average of the masses of all the naturally occurring isotopes of an element — recall that isotopes are different forms of an element that differ in the number of neutrons.
Because the mass in grams is too small for us to comprehend, we utilize atomic mass units (amu), where protons & neutrons have an amu of 1 while the amu of electrons is so low that it’s considered 0.
>> Molecular Weight:
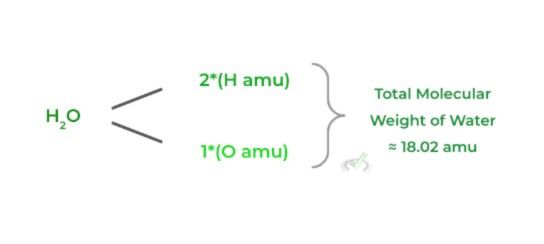
Basically the molecular equivalent of the atomic mass! To calculate the molecular weight, simply add all the atomic weights of all the elemental atoms in the molecule.
>> Mole:
By its formal definition, a mole is equal to 6.022 x 1023 molecular entities — these can be atoms, molecules, ions, etc. This is a little hard to explain in words and is better understood with a visual:
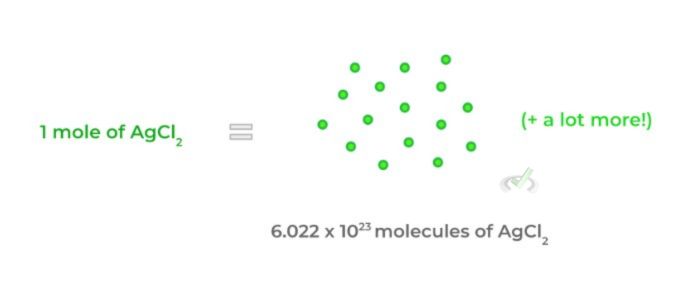
- It’s kinda like the way we use the term “dozen” — when we say a dozen eggs, we mean 12 eggs. Similarly, when we say 1 mole of H2O, it’s the same as saying there are 6.022 x 1023 molecules of water (but on a much larger scale!).
>> Molar Mass
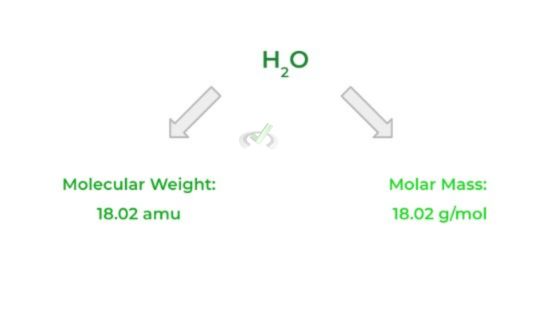
This term simply refers to the mass (in grams) of 1 mole of a substance. In other words, it’s the mass (in grams) of all the 6.022 x 1023 molecules, atoms, etc.
Luckily, the molar mass has the same numerical value as the molecular weight of a substance. The only thing that’s different is the units used: molecular weight uses amu, while molar mass will have g/mol.
Full Study Notes : Quantification of Matter
For more in-depth content review on quantification of matter, check out these detailed lesson notes created by top MCAT scorers.
2. Chemical Formulas of Molecules and Compounds
The chemical formulas of molecules and compounds tell us the elemental composition of the molecule/compound as well as the specific amount of atoms for each element and the ratio between the elements.
Specifically, there are 2 types of chemical formulas to know about for the MCAT: the molecular formula and the empirical formula. Let’s take a closer look at the main differences between the 2!
Simply put, the molecular formula tells you exactly how many of each atom is present in a molecule/compound, while the empirical formula shows the simplest whole number ratio of a molecule or compound. Look at the example for a better visual!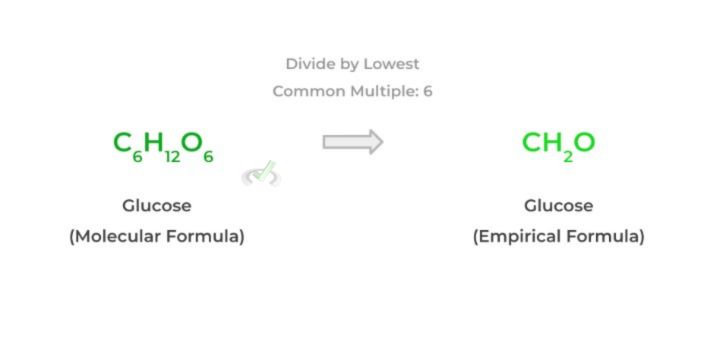
As shown above, we simply divide the subscripts of the molecular formula by the lowest common multiple, which is 6 (note: that the “1” subscript in the empirical formula is implied although not shown).
Another important thing to make note of is that the ratio of atoms to one another remains the same for both the molecular and empirical formula: it’s only the exact number of atoms (i.e. subscript) that’s different.
Full Study Notes : Chemical Formulas of Molecules and Compounds
For more in-depth content review on the differences between molecular and empirical formulas as well as some calculation examples, check out these detailed lesson notes created by top MCAT scorers.
3. Types of Chemical Reactions
Just like how we can categorize types of football plays — handoffs, quick passes, and play actions — we can also categorize chemical reactions into 5 main reactions as we’ll discuss below!
Additionally, it’s important to note that a lot of reactions there are other types of reactions that you may encounter in your general chemistry review, such as neutralization and redox reactions — however, these reactions also generally fall under one of these categories!A. Synthesis Reaction
- Occurs when 2 (or more) smaller molecules combine to form 1 big molecule!
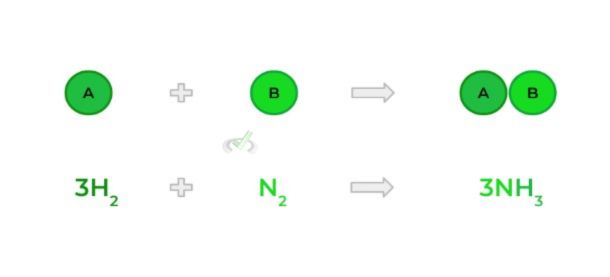
B. Decomposition Reaction
- Occurs when a bigger molecule breaks down to produce 2 (or more) smaller molecules; Essentially the opposite of a synthesis reaction!
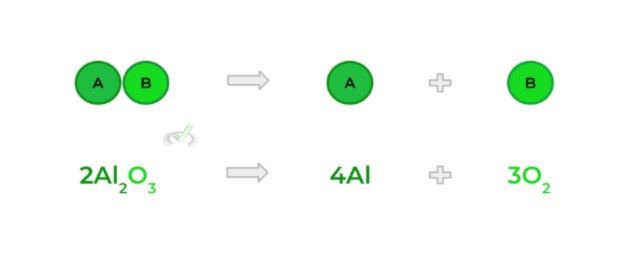
C. Combustion Reaction
Occurs when a substance reacts with O2, which results in a release of energy in the form of heat!
Combustion can occur with any reactant, but is primarily tested via the combustion of hydrocarbons!
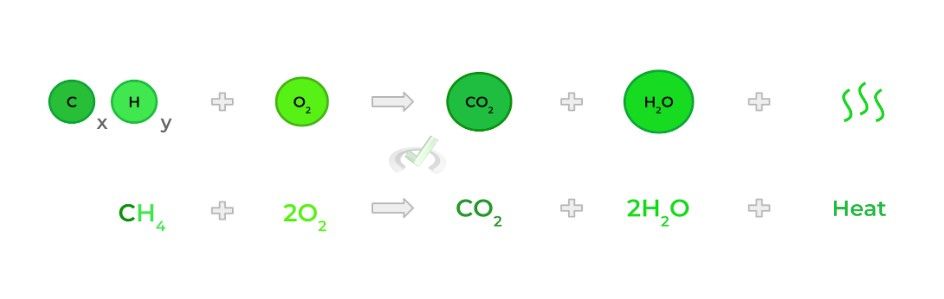
D. Single Substitution Reaction
Also called a single displacement reaction; occurs when 1 free element substitutes another similar element in a compound!
Usually, this involves an ionic compound, similar to a double substitution reaction, as we’ll discuss in a bit!
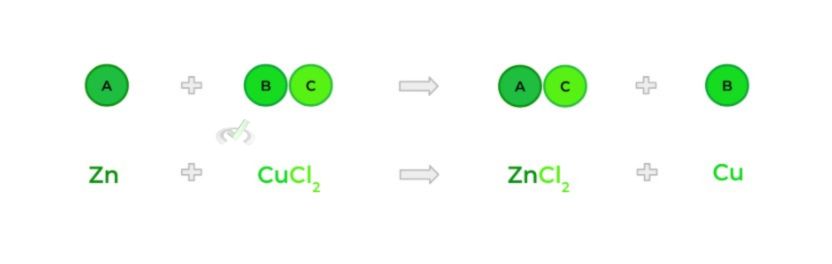
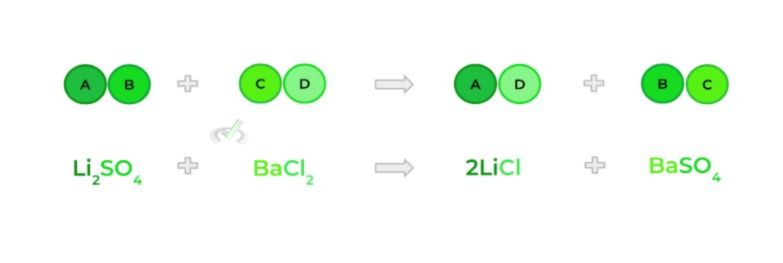
E. Double Substitution Reaction
- Also called a double displacement reaction; occurs when 2 ionic compounds swap their cations and anions — kinda like when NBA and NFL teams trade players!
Full Study Notes : Types of Chemical Reactions
For more in-depth content review on types of chemical reactions, check out these detailed lesson notes created by top MCAT scorers.
4. Conservation of Mass in Balancing Chemical Equations
One of the most fundamental skills that must be learned in order to tackle stoichiometric problems is the balancing of chemical equations in order to ensure that the number of atoms of each element remains constant for both sides!
This is essentially an application of the conservation of mass law which states that matter can neither be created nor destroyed — i.e. the amount of matter remains constant. Let’s look at the combustion of methane as an example!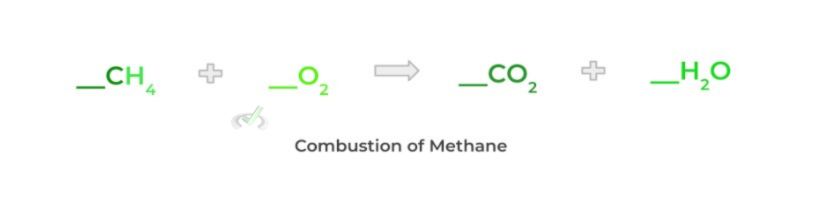
We now have to determine the stoichiometric coefficients that must be placed in front of the molecules! One way to keep track of all the atoms is via a chart, as shown below!
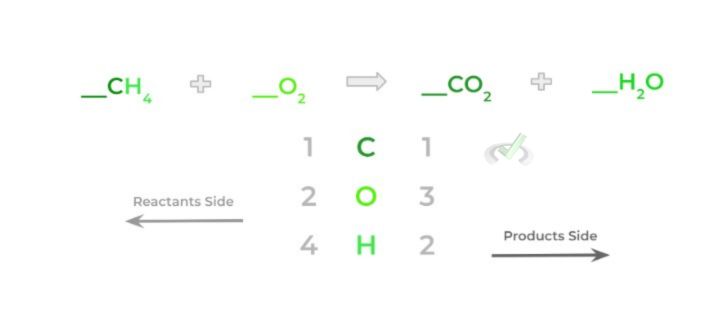
Now, let’s try to balance the equation! First, we see that the amount of carbon atoms remains the same for both sides (1) — we’ll leave those alone for now.
However, the hydrogen atoms are unbalanced: there are 4 hydrogen atoms from methane and 2 hydrogen atoms from the water molecule. Let’s go ahead and balance these by adding a 2 coefficient to the water molecule.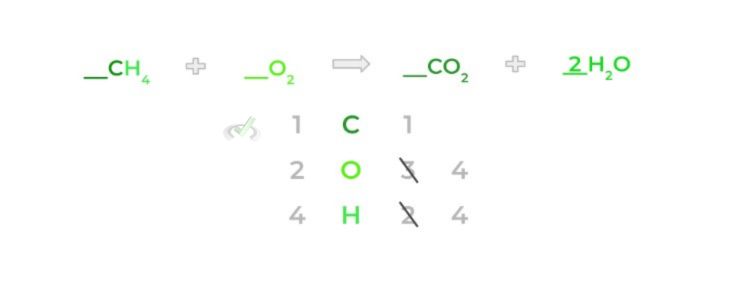
Notice how now there are 4 oxygen atoms on the product side because we also multiply the stoichiometric coefficient by the subscript as well. In addition, the amount of oxygen atoms on the product side increases to 4 oxygen atoms.
Finally, to balance out the oxygen atoms, add a 2 coefficient to the oxygen molecule on the reactants side — again, keep in mind that you’ll multiply the stoichiometric coefficient by the subscript.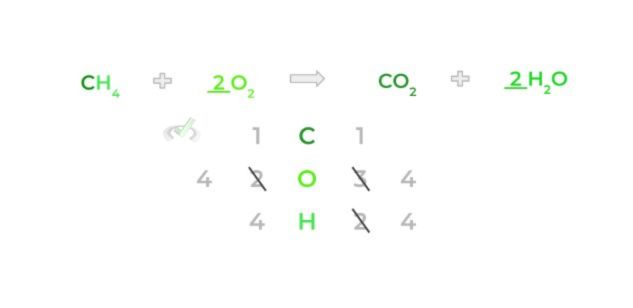
Now you have the complete balanced equation! Oftentimes, it may be beneficial to approach balancing equations with a “trial and error” strategy; however, we do have some tips and tricks for how to balance these equations in this article!
Full Study Notes : Conservation of Mass in Balancing Chemical Equations
For more in-depth content review on conservation of mass in balancing chemical equations, check out these detailed lesson notes created by top MCAT scorers.
5. Calculations of Chemical Equations
In most cases, the MCAT will test you on your ability to find the yield of a product of a chemical reaction given a certain amount of starting reactant. The key thing about chemical equations is that they give both the molecular and mole ratio between the products and reactants. Let’s again look at the combustion of methane!
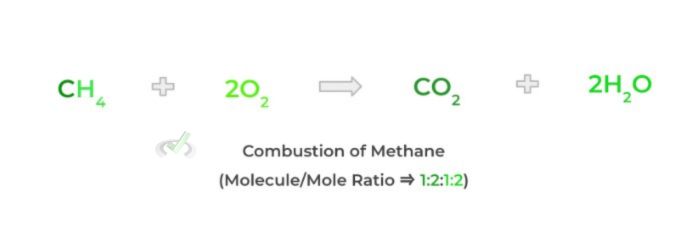
From the stoichiometric coefficients, we see that the molecule ratio is 1:2:1:2. This molecular ratio can be thought of in many different ways:
- “1 molecule of methane yields 1 molecule of carbon dioxide”
- “1 molecule of methane yields 2 molecules of water”
- “2 molecules of diatomic oxygen yields 1 molecule of carbon dioxide”
The mole ratio means that exact same thing except we’re using the unit “mole” instead of individual molecules. Recall that 1 mole is equal to 6.022 x 1023 molecular entities — in this case, we use molecules.
- “1 mole of methane (6.022 x 1023 molecules of methane) yields 1 mole of carbon dioxide (6.022 x 1023 molecules of carbon dioxide)”
- “2 moles of diatomic oxygen (6.022 x 1023 molecules of diatomic oxygen) yields 2 moles of water (6.022 x 1023 molecules of water)”
However, it’s important to note that this ratio does not hold true for mass units (i.e. grams) — this is because the atomic and molecular weights are different between molecules. So, saying 3.5 grams of methane yields 3.5 grams of carbon dioxide is incorrect.
To find the exact amount of grams for a product (like carbon dioxide) given a starting amount of a reactant (like 3. 5 grams of methane), we need to know the molar mass of the molecules and do a little bit of dimensional analysis. Below are the following steps:
1. First, find the molar mass of the reactants and products of interest. Recall that the molar mass is numerically equal to the molecular weight and uses the units g/mol.
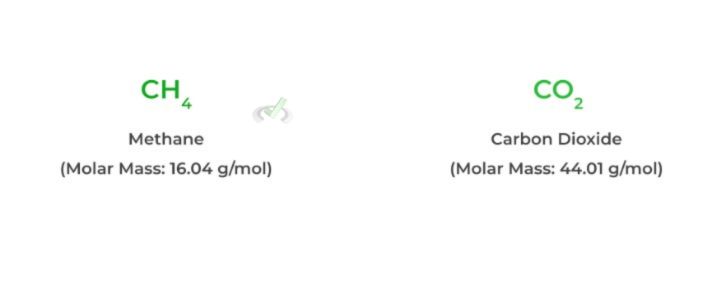
2. Next, multiply the mass of the reactant of interest by its molar mass as shown below! Notice how when you multiply the values, the “grams” units cancel out.
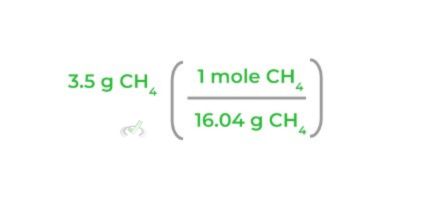
3. Then, multiply the equation by the corresponding mole ratio, making sure that the “moles CH4” will cancel each other out as shown below:
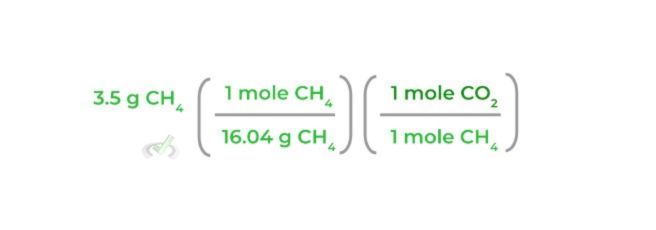
4. After that, multiply the equation by the molar mass of CO2 again, making sure that the “moles CO2” will cancel out each other out as shown below:
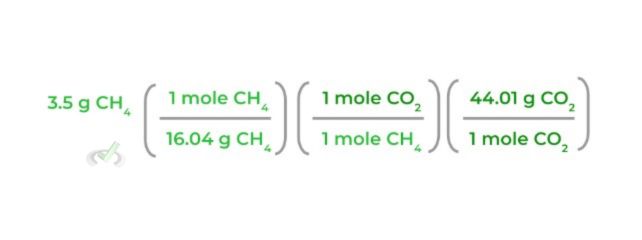
5. Finally, multiply all the values on the numerator together and those on the denominator together and then simplify to get grams of CO2!
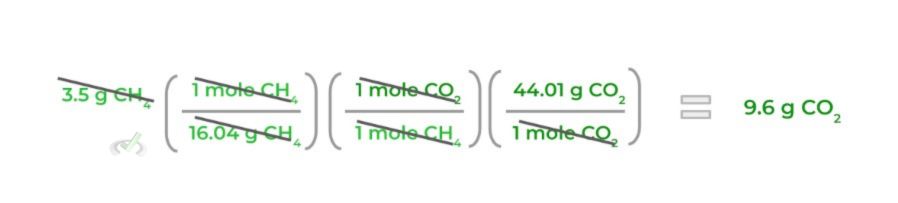
As shown, a big part of the stoichiometric calculations of chemical reactions is dimensional analysis. Always make sure that the correct values cancel out at the end of the calculation!
Full Study Notes : Calculations of Chemical Equations
For more in-depth content review on stoichiometric calculations of chemical equations, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about stoichiometry in general chemistry!
Term | Definition |
|---|---|
Atomic Weight | The average of the masses of all the naturally occurring isotopes of an element; Utilizes the units amu |
Molecular Weight | The summation of the atomic weights of a molecule |
Mole | Equal to 6.022 x 1023 molecular entities — molecules, atoms, ions, etc. |
Molar Mass | Value which refers to the mass of 1 mole of any substance; Numerically equivalent to the molecular weight |
Molecular Formula | A type of chemical formula that tells you the exact amount of each type of elemental atom in a molecule |
Empirical Formula |
A type of chemical formula which gives you the lowest whole number ratio of the atoms |
Synthesis Reaction |
A type of reaction where 2 (or more) smaller molecules combine to form 1 bigger molecule |
Decomposition Reaction |
A type of chemical reaction where 1 big molecule breaks down to produce 2 (or more) smaller molecules |
Combustion Reaction |
A type of chemical reaction where a substance reacts with oxygen to release energy in the form of heat |
Single Substitution Reaction |
A type of chemical reaction where a free element substitute another similar element in a compound |
Double Substitution Reaction |
A type of chemical reaction where 2 ionic compounds swap their anions and cations |
Additional FAQs - Stoichiometry on the MCAT
What are the 5 Steps of Stoichiometry? – MCAT
1. Identify the molar mass of the reactants and products involved.
2. Multiply the molar mass by the grams of the reactant of interest.
3. Next, multiply the equation by the corresponding mole ratio.
4. Then, multiply the equation by the molar mass of the product of interest.
5. Finally, multiply the values of the numerator and the values of the denominator and simplify to get the grams of the product.
What are the 4 Steps of Stoichiometry? – MCAT
1. Identify the molar mass of the reactants and products involved and multiply the molar mass by the grams of the reactant of interest.
2. Next, multiply the equation by the corresponding mole ratio.
3. Then, multiply the equation by the molar mass of the product of interest.
4. Finally, multiply the values of the numerator and the values of the denominator and simplify to get the grams of the product.
Additional Reading Links – Study Notes for Stoichiometry on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Acids and Bases on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot