Curious about how scientists can determine the structure of a molecule without seeing it directly? Or how do they identify unknown substances with just a tiny sample?
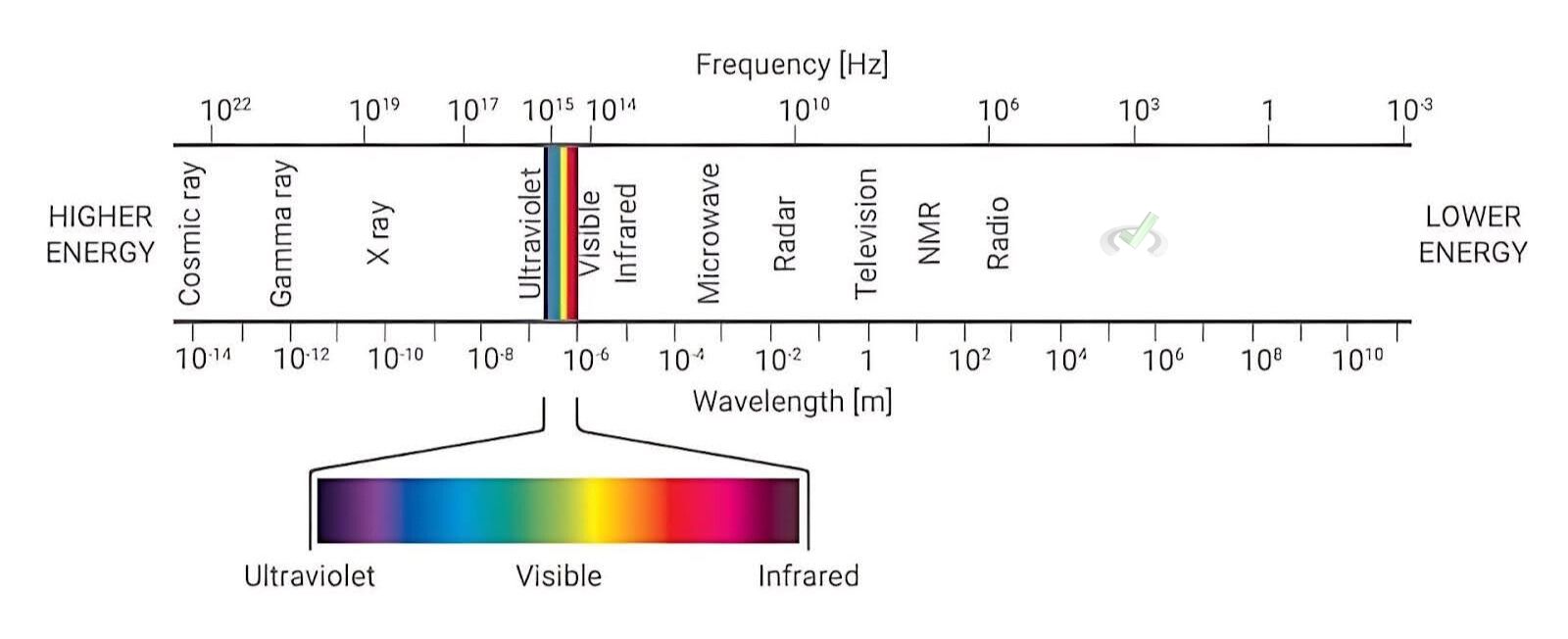
The answer lies in chemical spectroscopy. It is a powerful technique that uses light to reveal the secrets of molecules. Let's analyze how molecules interact with different types of electromagnetic radiation in detail.
I. Introduction to Chemical Spectroscopy
Chemical spectroscopy uses electromagnetic radiation to study the structure and properties of molecules. When molecules absorb or emit light, they produce a spectrum. It is like a molecular fingerprint.
This spectrum can reveal detailed information about the molecule's structure and composition. Spectroscopy is widely used in environmental science, medicine, and materials science. This study helps analyze and monitor substances.
II. Types of Spectroscopy
Infrared (IR) Spectroscopy
IR spectroscopy measures the vibrations of molecules. Different bonds absorb infrared light at different wavelengths. It creates a spectrum that can identify functional groups within a molecule.
Example: A C-H bond will absorb at a wavelength different from an O-H bond.
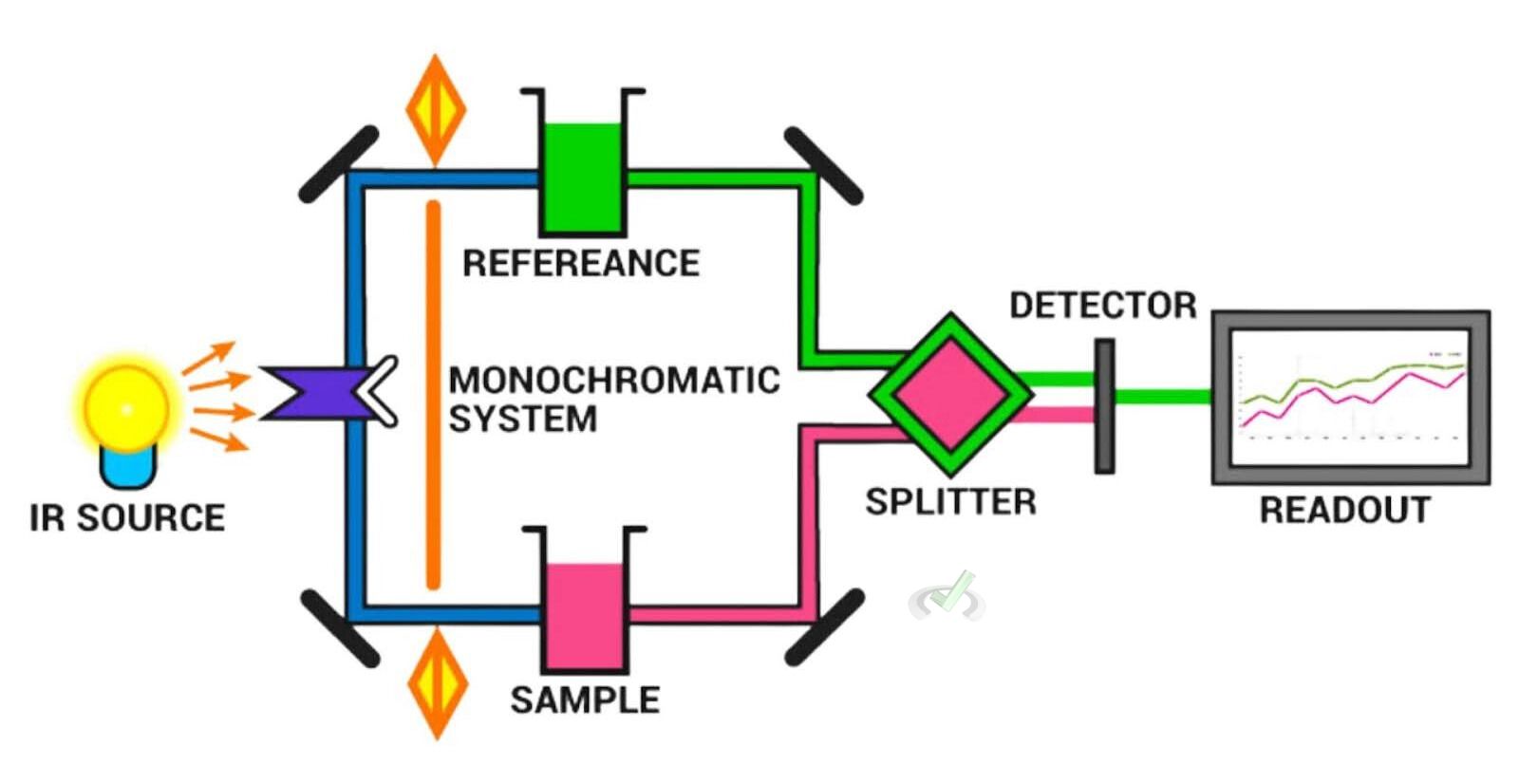
How IR Spectroscopy Works
- Sample Preparation: The sample is prepared so that it can interact with infrared light. This is often done by creating a thin film or a pellet.
- Light Interaction: Infrared light is passed through the sample.
- Absorption Measurement: The sample absorbs some of the light. Then, the detector measures how much light is absorbed at each wavelength.
- Spectrum Analysis: The resulting spectrum shows peaks where light is absorbed. It corresponds to different bond vibrations.
Functional Group Absorption Ranges:
- O-H stretch: 3200-3600 cm⁻¹
- C=O stretch: around 1700 cm⁻¹
- C-H stretch: around 3000 cm⁻¹
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy utilizes the magnetic properties of certain atomic nuclei. It is particularly useful for determining the structure of organic compounds and provides information about a molecule's carbon-hydrogen framework.
Example: Hydrogen atoms in different environments (e.g., CH3 vs. CH2) will produce different signals
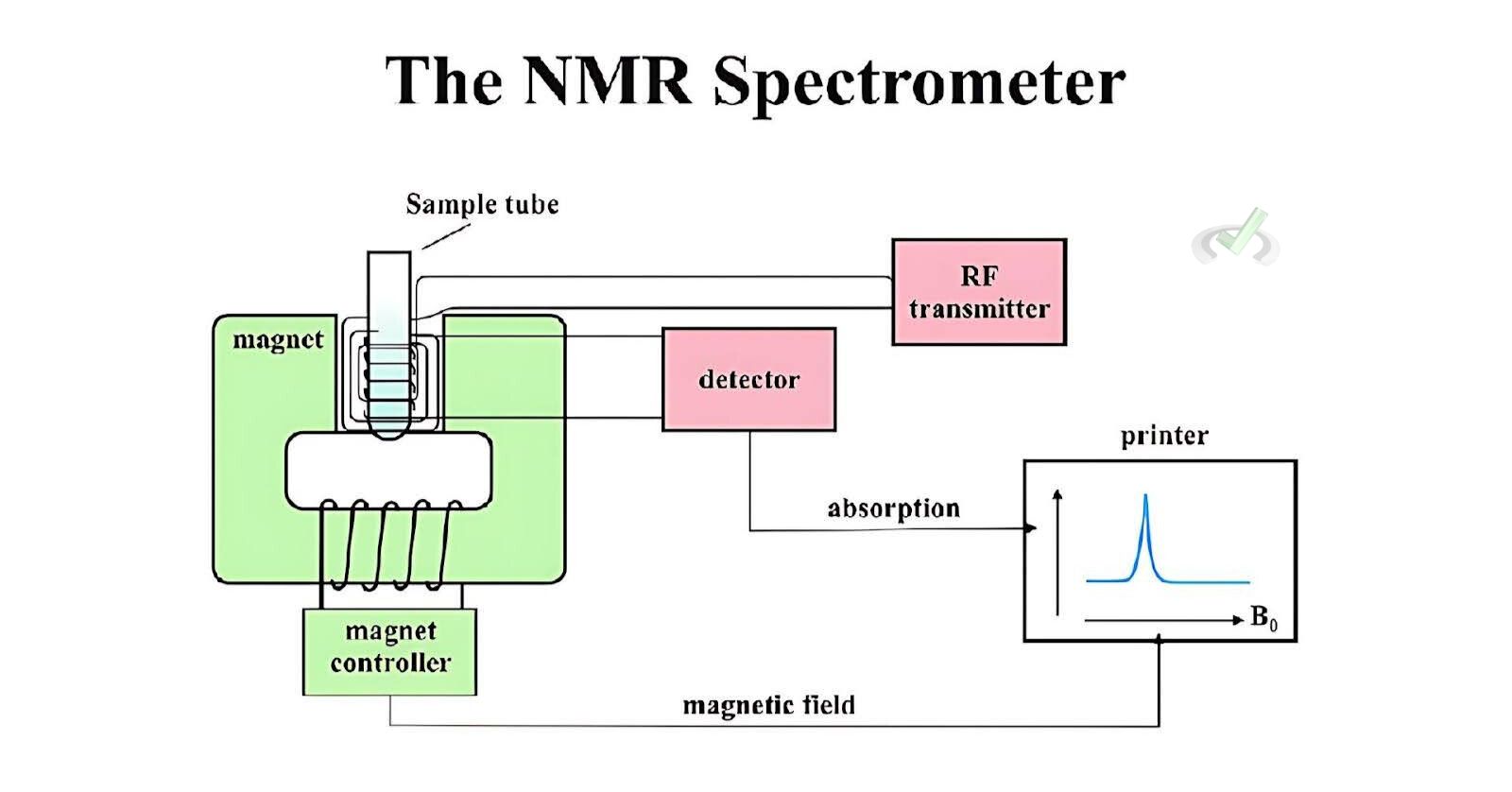
How NMR Spectroscopy Works
- Sample Preparation: The sample is dissolved in a solvent that does not interfere with the measurement.
- Magnetic Field Application: The sample is placed in a strong magnetic field.
- Radiofrequency Pulse: A radiofrequency pulse is applied. It causes the nuclei to absorb energy and change their spin state.
- Signal Detection: As the nuclei return to their original spin state, they emit energy. This is detected and transformed into a spectrum.
Types of NMR:
- 1H1H NMR (proton NMR): Provides information about hydrogen atoms in a molecule.
- 13C13C NMR (carbon-13 NMR): Provides information about carbon atoms in a molecule.
Chemical Shift:
- The position of NMR signals on the spectrum is measured in parts per million (ppm). It indicates the environment of the nuclei. The common ranges are 0-10 ppm for 1H1H NMR and 0-200 ppm for 13C13C NMR.
Ultraviolet-Visible (UV-Vis) Spectroscopy
UV-Vis spectroscopy measures the absorption of ultraviolet or visible light by a molecule. This technique is often used to study compounds with conjugated systems. It is where electrons can move more freely across several atoms.
Example: The more conjugated a system is, the longer the wavelength of light it can absorb.
How UV-Vis Spectroscopy Works
- Sample Preparation: The sample is usually in solution form.
- Light Interaction: UV or visible light is passed through the sample.
- Absorption Measurement: The detector measures how much light is absorbed at each wavelength.
- Spectrum Analysis: The spectrum shows peaks where light is absorbed. It indicates electronic transitions within the molecule.
Significance of Peaks:
- Peaks in the UV-Vis spectrum correspond to electronic transitions. The wavelength of the peak can give information about the extent of conjugation in the molecule.
III. Interpreting Spectra
Infrared (IR) Spectrum
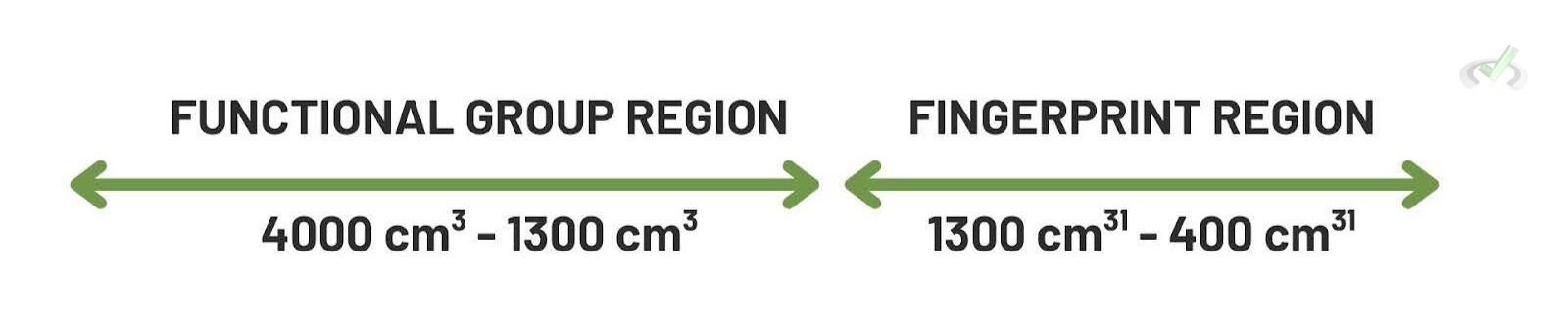
- Fingerprint Region: The area from 600-1500 cm⁻¹ contains many peaks and is unique to each molecule.
- Functional Group Region: The area above 1500 cm⁻¹ helps identify functional groups. For instance, O-H stretches appear around 3200-3600 cm⁻¹. Then, C=O stretches appear around 1700 cm⁻¹.
Nuclear Magnetic Resonance (NMR) Spectrum
- Chemical Shift: The position of NMR signals on the spectrum is measured in parts per million (ppm). It gives information about the environment of the nuclei.
- Integration: The area under each signal indicates the number of nuclei contributing to that signal.
- Multiplicity: The splitting of signals into multiple peaks (singlet, doublet, triplet). It indicates the number of neighboring nuclei.
Ultraviolet-Visible (UV-Vis) Spectrum
- Absorption Peaks: The peaks in the UV-Vis spectrum indicate electronic transitions.
- Molar Absorptivity: The height of the peaks gives information about the concentration of the absorbing species.
IV. Applications of Chemical Spectroscopy
Identifying Unknown Compounds
Chemical spectroscopy is essential in identifying unknown compounds. By comparing the spectrum of an unknown sample to reference spectra, chemists can determine the sample's structure and composition.
Studying Chemical Reactions
Spectroscopy can monitor the progress of chemical reactions. For instance, NMR can show changes in the environment of hydrogen atoms as reactants turn into products.
Example: A reaction where an alkene is converted to an alcohol. IR spectroscopy can show the disappearance of C=C stretches and the appearance of O-H stretches.
Quality Control in Manufacturing
In industries, spectroscopy ensures the quality and purity of products. For example, IR spectroscopy can check for impurities in pharmaceuticals.
V. Connecting Chemical Spectroscopy to Broader Scientific Concepts
Chemical spectroscopy is crucial not only in organic chemistry but also in various scientific disciplines and practical applications.
Spectroscopy and Astrophysics
Spectroscopy is used in astrophysics to analyze the composition of stars and planets. Scientists can determine their chemical makeup by studying the light emitted or absorbed by celestial objects.
Spectroscopy and Environmental Science
Environmental scientists use spectroscopy to monitor pollutants. For instance, UV-Vis spectroscopy can detect contaminants in water. It does this by measuring the absorption of specific wavelengths.
Spectroscopy in Medicine
In medicine, spectroscopy helps in diagnose diseases. For example, NMR spectroscopy forms the basis of MRI (Magnetic Resonance Imaging). It is a crucial tool for non-invasive medical imaging.
Example: MRI uses the principles of NMR to create detailed images of organs and tissues. It helps doctors diagnose and monitor various conditions.
VI. Wrap-Up and Key Terms
Understanding chemical spectroscopy involves several key concepts:
- Electromagnetic Radiation: Waves of electric and magnetic fields that propagate through space.
- Spectrum: A range of wavelengths produced by the absorption or emission of light.
- Infrared (IR) Spectroscopy: Measures molecular vibrations.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Uses magnetic properties of nuclei to determine molecular structure.
- Ultraviolet-Visible (UV-Vis) Spectroscopy: Measures absorption of UV and visible light.
VII. Practice Questions
Sample Practice Question 1
Which type of spectroscopy measures molecular vibrations?
A. NMR Spectroscopy
B. UV-Vis Spectroscopy
C. IR Spectroscopy
D. Mass Spectroscopy
Ans. C
IR spectroscopy measures the vibrations of molecules. It helps identify different functional groups.
Sample Practice Question 2
What does NMR spectroscopy primarily provide information about?
A. Electronic transitions
B. Molecular vibrations
C. Carbon-hydrogen framework
D. Molecular mass
Ans. C
NMR spectroscopy provides detailed information about the carbon-hydrogen framework of organic compounds. It analyzes the magnetic properties of atomic nuclei.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
