Aldehydes and ketones are important organic compounds. Understanding their structures and naming conventions is essential for studying organic chemistry. This guide covers the basics, including chemical equations and structural formulas, to help you grasp these concepts.
I. Introduction to Aldehydes and Ketones
A. What are Aldehydes and Ketones?
Aldehydes and ketones are organic molecules containing a carbonyl group (C=O). The carbonyl group is comprised of a carbon atom double-bonded to an oxygen atom. This group is highly reactive and is key to the properties of these compounds.
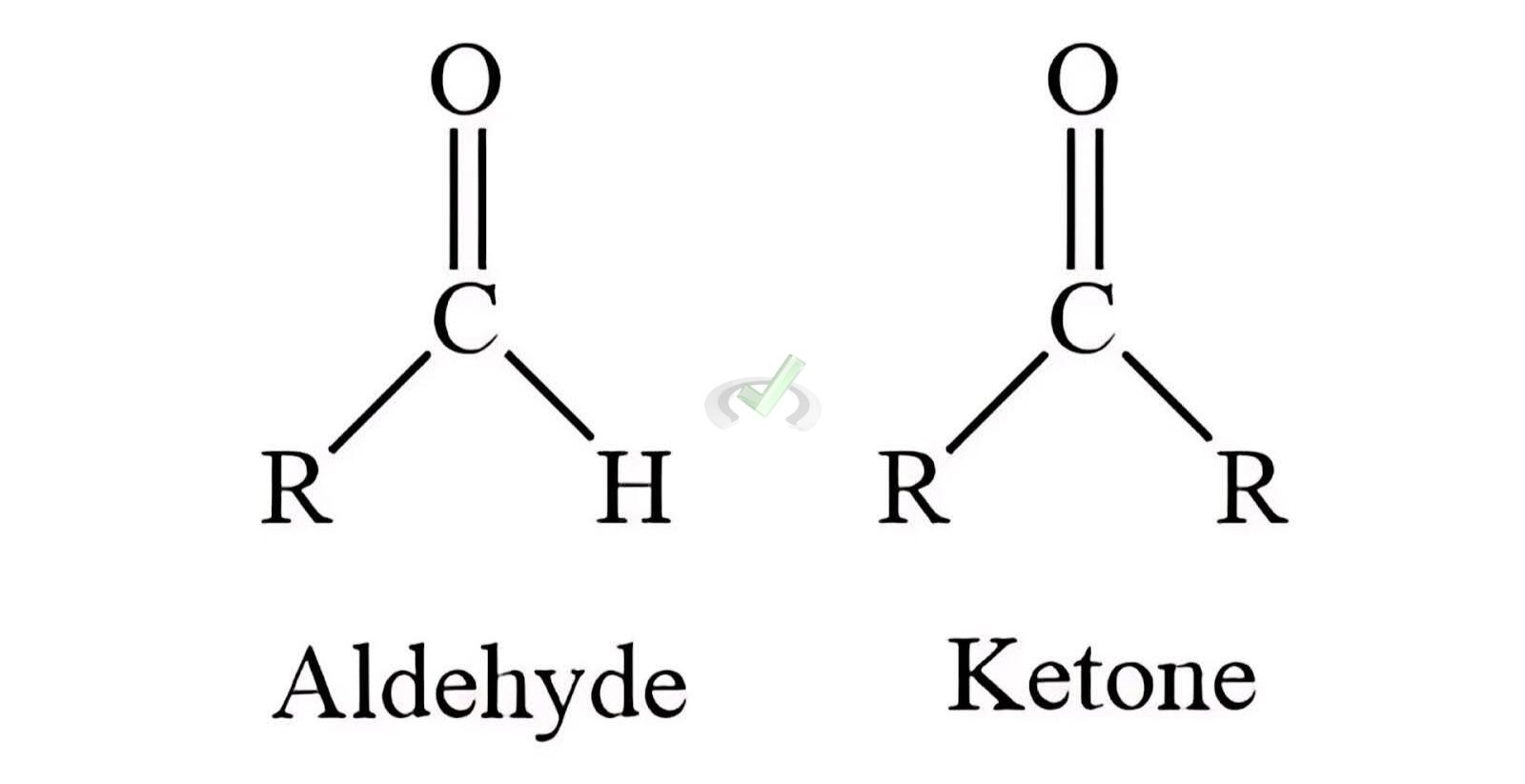
- Aldehydes have the carbonyl group at the end of the carbon chain. The general formula is R-CHO, where R represents a hydrocarbon group or hydrogen.
- Ketones have the carbonyl group within the carbon chain. The general formula is R-CO-R', where R and R' are hydrocarbon groups.
B. Importance of Aldehydes and Ketones
Aldehydes and ketones are prevalent in both natural and synthetic compounds. They are crucial in metabolic pathways, such as glycolysis and the citric acid cycle. They are also key in industrial processes like the production of solvents and perfumes.
II. Structure of Aldehydes and Ketones
A. Structural Formula
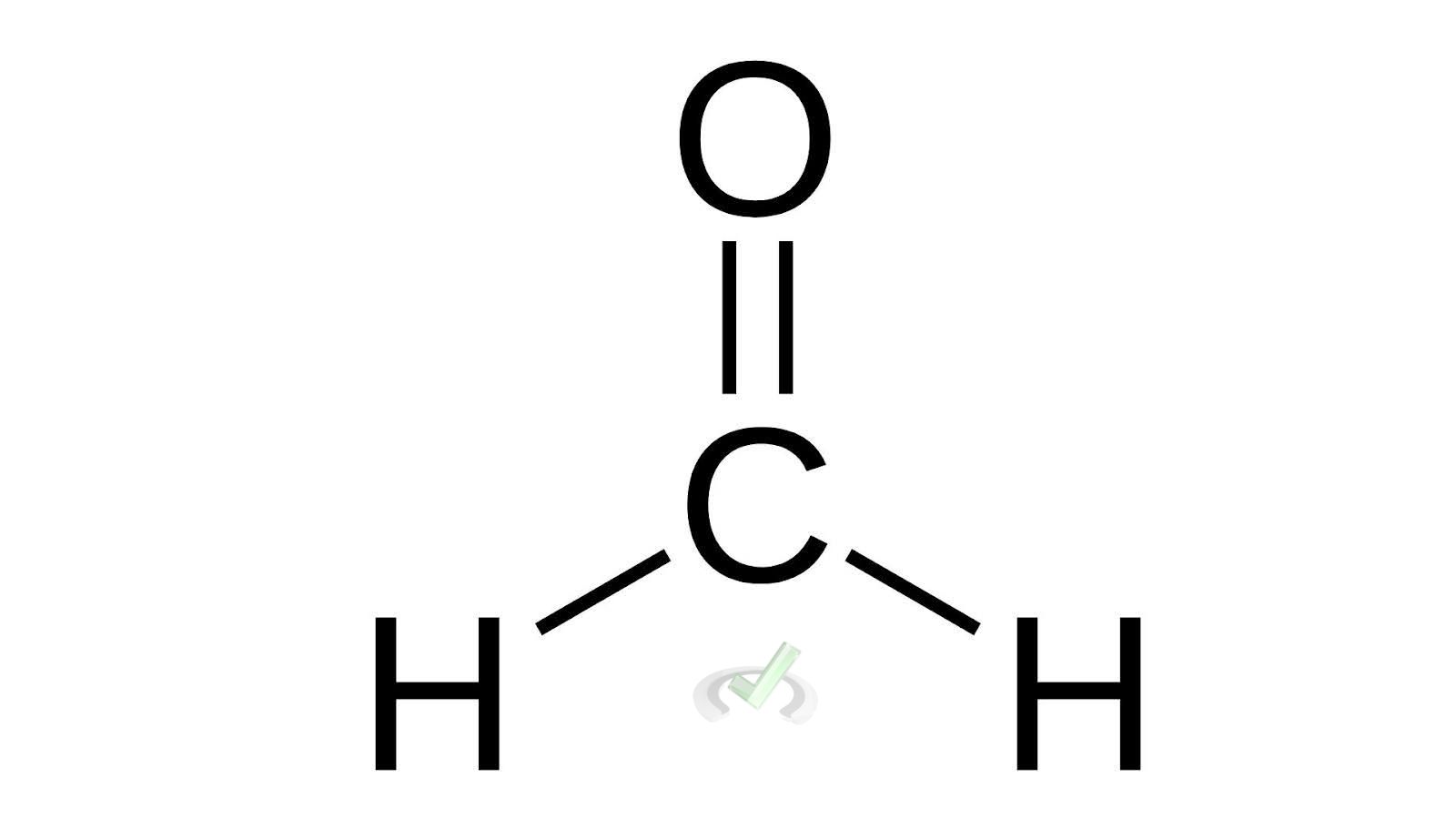
- Aldehyde Example: Formaldehyde (CH₂O)
Formaldehyde has a simple structure with one carbon atom double-bonded to an oxygen atom and single-bonded to two hydrogen atoms.
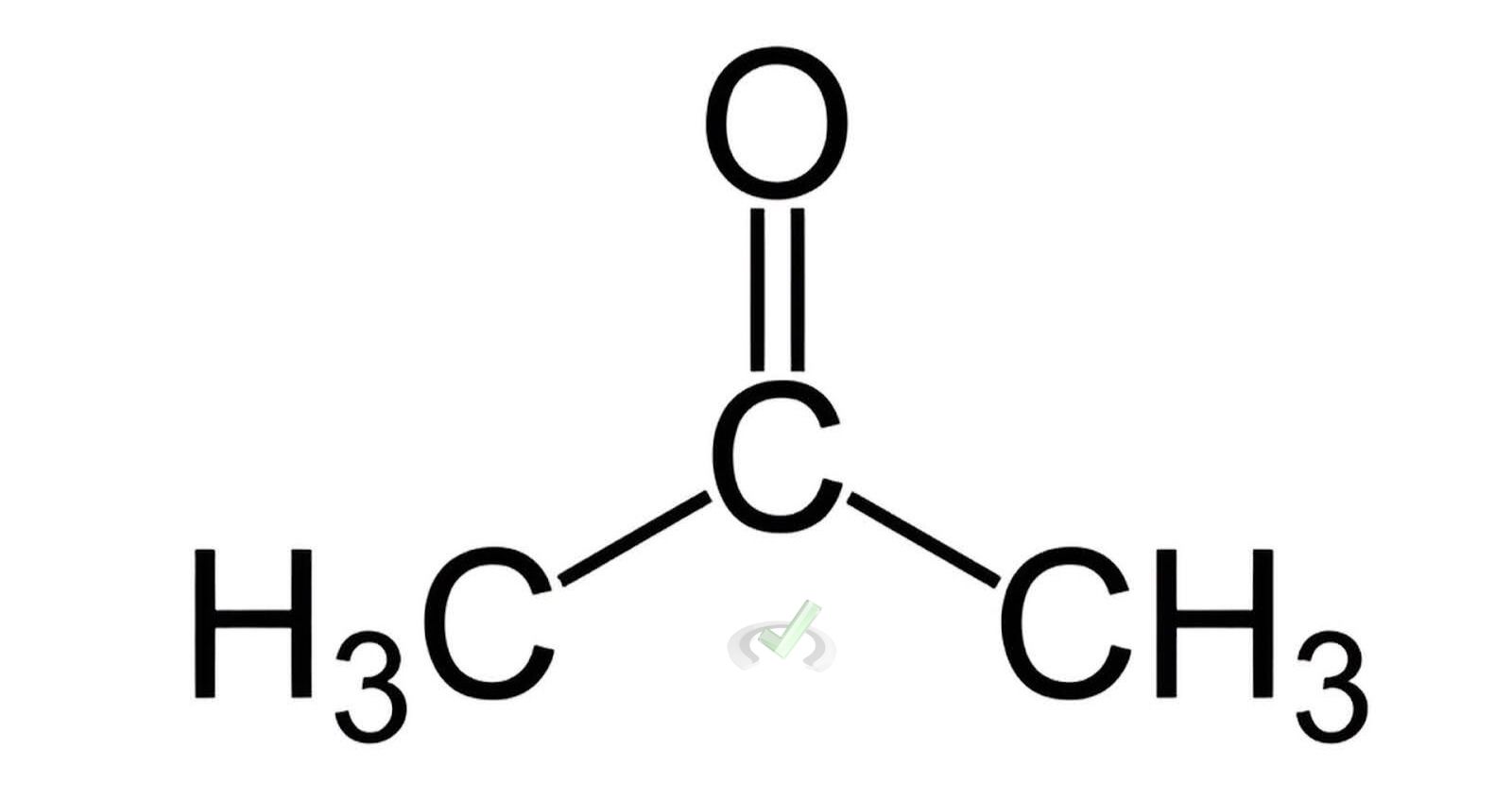
- Ketone Example: Acetone (C₃H₆O)
Acetone has a three-carbon chain with the middle carbon double-bonded to an oxygen atom.
III. Nomenclature of Aldehydes and Ketones
A. Naming Aldehydes
To name aldehydes, follow these steps:
- Identify the longest carbon chain containing the carbonyl group.
- Replace the -e ending of the parent alkane with -al.
- Number the carbon chain starting from the carbonyl carbon as position 1.
Example:
- Methanal (formaldehyde): H-CHO
- Ethanal (acetaldehyde): CH₃-CHO
B. Naming Ketones
To name ketones, follow these steps:
- Identify the longest carbon chain containing the carbonyl group.
- Replace the -e ending of the parent alkane with -one.
- Number the carbon chain so the carbonyl carbon gets the lowest possible number.
- Indicate the position of the carbonyl group.
Example:
- Propanone (acetone): CH₃-CO-CH₃
- Butanone: CH₃-CO-CH₂-CH₃
IV. Chemical Reactions Involving Aldehydes and Ketones
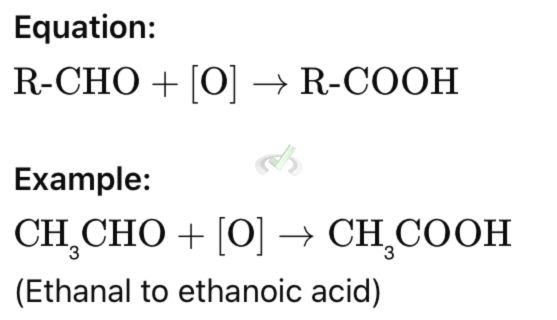
A. Oxidation of Aldehydes
Aldehydes can be oxidized to carboxylic acids. This involves adding an oxygen atom to the carbonyl carbon.
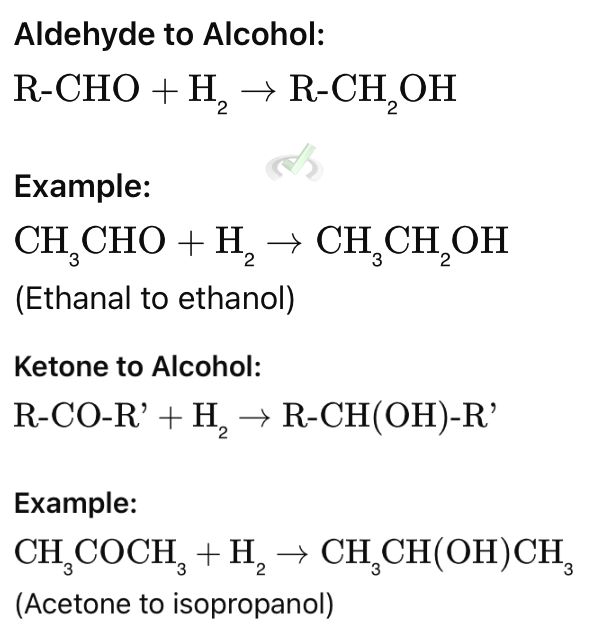
B. Reduction of Aldehydes and Ketones
Both aldehydes and ketones can be reduced to alcohols by adding hydrogen atoms.
V. Applications and Relevance
A. Biological Systems
Aldehydes and ketones play significant roles in biological systems. For instance, during glycolysis, glucose is broken down into pyruvate, a key ketone intermediate. Pyruvate then enters the citric acid cycle, contributing to cellular respiration and energy production.
B. Industrial Uses
Aldehydes and ketones are vital in various industrial applications. Formaldehyde, for example, is extensively used in making polymers and resins. Ketones like acetone are essential solvents in the production of pharmaceuticals and cosmetics.
C. Health and Safety
Aldehydes and ketones can be hazardous. Formaldehyde is toxic and a known carcinogen. Acetone is flammable and can cause irritation if inhaled or in contact with the skin. Proper handling and storage are crucial.
VI. Connecting Aldehydes and Ketones to Broader Organic Chemistry Concepts
Understanding aldehydes and ketones is essential for broader organic chemistry, including topics relevant to the MCAT.
A. Synthesis and Functional Group Transformations
Aldehydes and ketones are often used as intermediates in organic synthesis. For example, converting an alcohol to a carboxylic acid involves two oxidation steps, with an aldehyde as an intermediate.
B. Reaction Mechanisms
The reactivity of the carbonyl group is central to many organic reactions. For instance, nucleophilic addition reactions, where nucleophiles like water or alcohols add to the carbonyl carbon, are foundational mechanisms. Recognizing these patterns helps in predicting products and intermediates in reaction mechanisms.
C. Biochemical Relevance
Enzymes such as alcohol dehydrogenase convert ethanol to acetaldehyde, highlighting the importance of these compounds in detoxification processes in the liver. Understanding enzyme-catalyzed reactions involving aldehydes and ketones extends to broader topics like enzyme kinetics and metabolic regulation.
D. Spectroscopy and Identification
Spectroscopy is a technique used to identify compounds. Aldehydes and ketones have characteristic peaks in IR spectroscopy (around 1700 cm⁻¹ for the C=O stretch).
NMR spectroscopy can further distinguish between aldehydes and ketones based on their unique chemical environments. These analytical techniques are crucial for structure determination.
E. Tautomerism
Aldehydes and ketones can exist in equilibrium with their enol forms, a concept known as keto-enol tautomerism. This is important in understanding reactivity and stability in various reactions.
F. Physical Properties
Aldehydes and ketones have distinct physical properties. They generally have higher boiling points than hydrocarbons due to the polarity of the carbonyl group.
However, their boiling points are lower than alcohols because they lack hydrogen bonding. They are also soluble in water, especially the lower molecular weight compounds, due to their ability to form hydrogen bonds with water molecules.
VII. Wrap-Up and Key Terms
Key Terms
- Carbonyl Group (C=O): A carbon atom double-bonded to an oxygen atom.
- Aldehyde (R-CHO): An organic compound with a carbonyl group at the end of the carbon chain.
- Ketone (R-CO-R'): An organic compound with a carbonyl group within the carbon chain.
- Oxidation: A chemical reaction where a molecule loses electrons or gains oxygen.
- Reduction: A chemical reaction where a molecule gains electrons or hydrogen.
- Tautomerism: The ability of aldehydes and ketones to exist in equilibrium with their enol forms.
- Spectroscopy: Techniques used to identify compounds based on their interaction with electromagnetic radiation.
VIII. Practice Questions
Sample Practice Question 1
Identify the following compound: CH₃CH₂CHO
A. Propanone
B. Propanal
C. Butanal
D. Butanone
Ans. B
The compound has three carbons with the carbonyl group at the end, making it an aldehyde.
Sample Practice Question 2
What is the product of the reduction of butanone?
A. Butanol
B. Butanoic acid
C. Butane
D. Butanal
Ans. A
Reduction of butanone (a ketone) results in a secondary alcohol, butanol.
Understanding aldehydes and ketones is crucial for grasping more complex organic chemistry topics. Keep practicing and exploring their reactions to deepen your knowledge!







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
