From the zesty taste of a glass of lemon water to the bitterness of a lot of laundry detergents, we pretty much encounter acids and bases on a daily basis from typical items and products we may have in our household.
We can give a safe bet that you may have encountered and had a couple of weeks devoted to this topic in your General Chemistry II class! This is because acids and bases are an important topic not just in the realm of general chemistry, but also looking at their physiological applications!
This chapter overview should give you a good foundation for the basics of acids and bases before going into our in depth articles. Let’s go ahead and get started!
Acids and Bases on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
This is definitely one of the more high yield sections on the MCAT and, as such, will be one that’s likely more tested on.
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Acids and Bases
When approaching content review for acids and bases, try to also align your review with your study of solubility, especially paying close attention to solubility equilibria as this serves as the foundation for understanding the dissociation of strong and weak acids (and bases)
Additionally, as we’ll see when we approach values such as pH, pOH, pKa, and pKb, try to also refresh your study on log functions — although a little daunting at first, mastering the basics of simple logs will help you not just here but in other MCAT topics as well, especially physics!
1. Main Definitions of Acids and Bases
There are 3 main definitions that have been used in order to categorize a molecule as an acid or base: Arrhenius, Bronsted-Lowry, and the Lewis definitions of acids and bases. Below are some straightforward explanations for each definition:
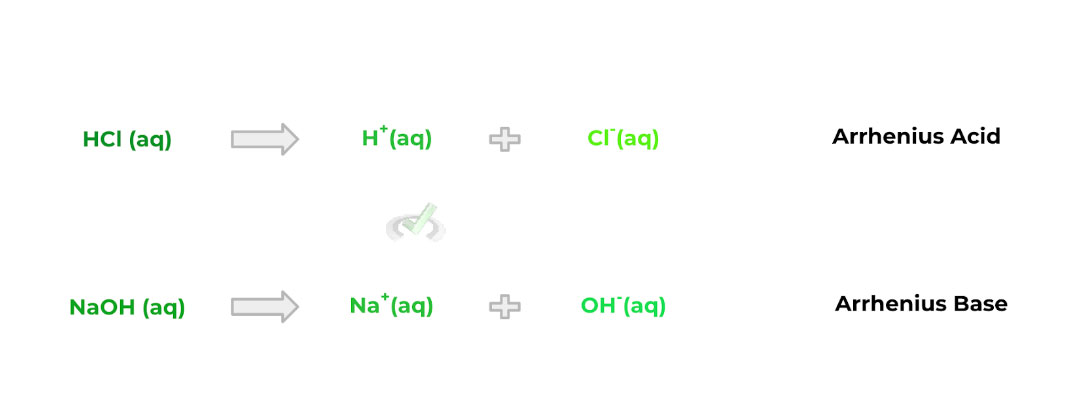
A. Arrhenius
Compared to the other acid/base definition, the Arrhenius classification is probably the simplest, but is also the most restricted in regards to what can be classified as an acid/base when using this definition.
Acid: A molecule that produces a proton (H+) upon dissociation
Base: A molecule that produces a hydroxide ion (OH-) upon dissociation
B. Bronsted-Lowry
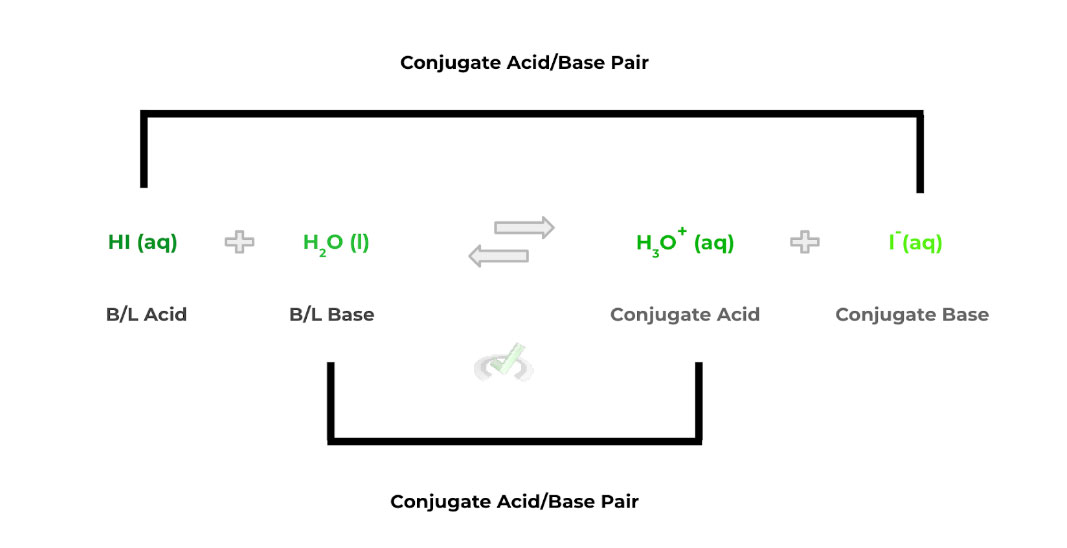
The interesting thing to note about a Bronsted-Lowry acid/base is that it involves a proton transfer between a Bronsted-Lowry acid and base.
Additionally, after the proton transfer occurs, the B/L acid and base also have a conjugate base and acid that forms, respectively — in the reverse reaction, the conjugate acid and base act in the same way.
Acid: Proton Donor
Base: Proton Acceptor
C. Lewis
In contrast with the Arrhenius definition of acids and bases, this definition is the most lenient in regards to what’s considered an acid or base. This definition will also be the best to refer to when talking about nucleophiles and electrophiles (hint, hint…..).
Acid: Electron Acceptor
Base: Electron Donor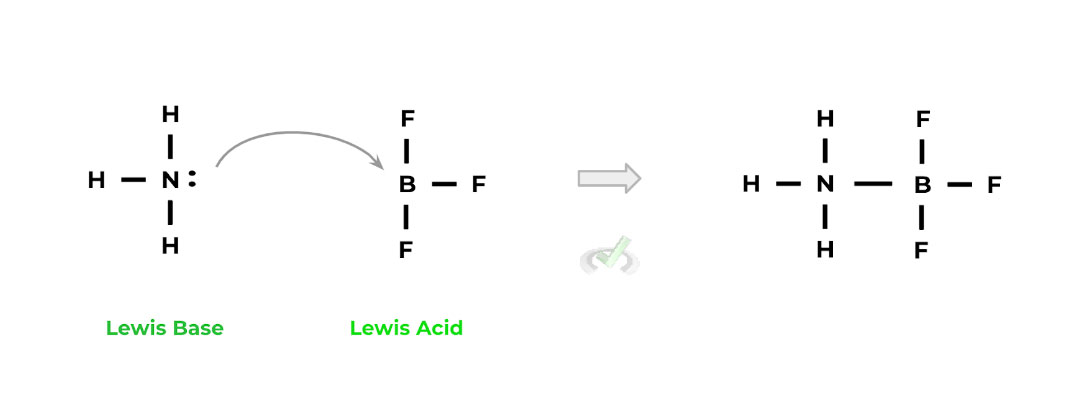
Full Study Notes : Main Definitions of Acids and Bases on the MCAT
For more in-depth content review on the main definitions of acids and bases that are used, check out these detailed lesson notes created by top MCAT scorers.
2. Acid and Bases Nomenclature
Before getting to the main reactions of acids and bases, let’s go ahead and get into the basic naming of acids and bases. Generally speaking, and for most of the time in the MCAT, acids will be formed by a covalent bond between a proton (H+) and an anion.
Usually, the anions that bind with H+ to form an acid will generally end in the suffix “-ide.” In order to get the new suffix you’ll use in the acid name, simply replace the “-ide” of the anion with “-ic”: for example, bromide (Br-) becomes bromic.
When combined together, the acid name will start with the prefix “-hydro” and end with the “-ic” suffix of whatever anion formed the acid, followed by the word “acid.” Take a look at how the names of strong acids are formed as shown below: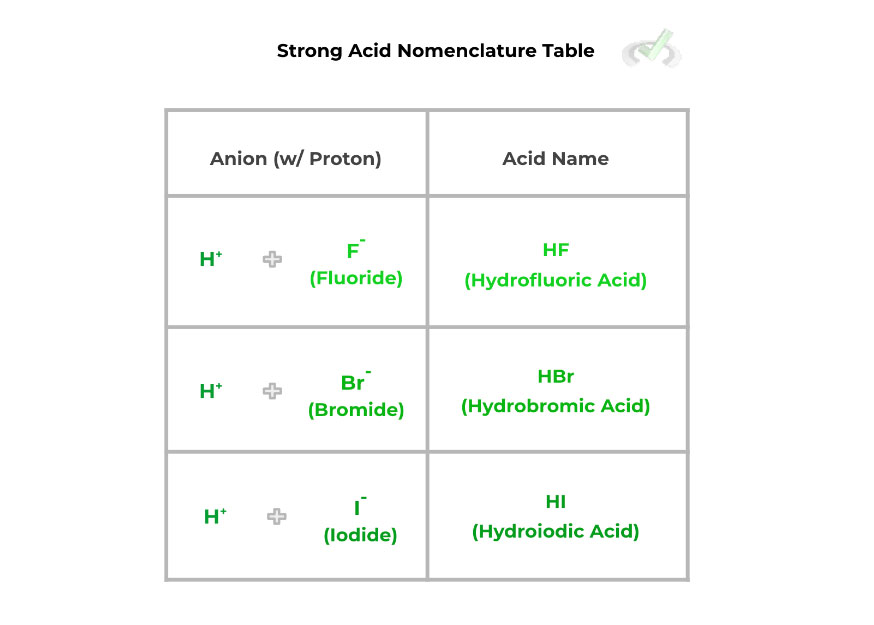
There’s another special class of acids called oxyacids which are basically acids that contain one or more oxygen atoms. As such, they are derived from oxyanions which often end in the suffixes “-ate” and “-ite,” which simply means they contain more or less oxygen atoms, respectively.
Oxyacids follow a similar naming pattern to regular anions with just a little slight twist: oxyanions ending in the “-ate” suffix will be replaced with a “-ic” suffix, while those ending in the “-ite” suffix with the “-ous” suffix as shown below!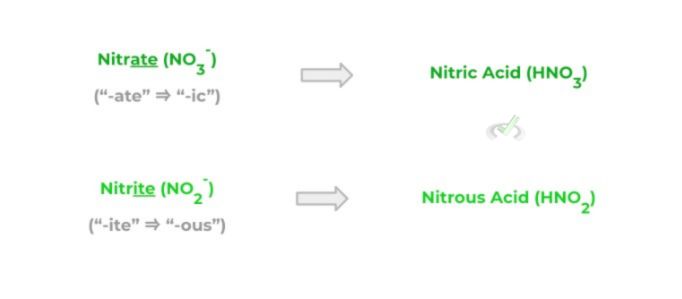
The naming of bases is pretty straightforward as each base will contain a hydroxide ion bonded to a cation: you simply name the base with the cation name followed by hydroxide. For example, NaOH is sodium hydroxide. Note that for acid naming, we’re primarily restricting it to the context of Arrhenius's definition.
Full Study Notes : Acid and Bases Nomenclature
For more in-depth content review on the basics of the nomenclature of acids and bases, check out these detailed lesson notes created by top MCAT scorers.
3. Acid/Base Dissociation and Relation to Acid/Base Strength
Interestingly, acids and bases can also be categorized based on their “strength” into whether they are strong or weak acid/base. In the simplest sense, strong acids/bases will completely dissociate into hydrogen and hydroxide ions, respectively. Take a look at the equations below:
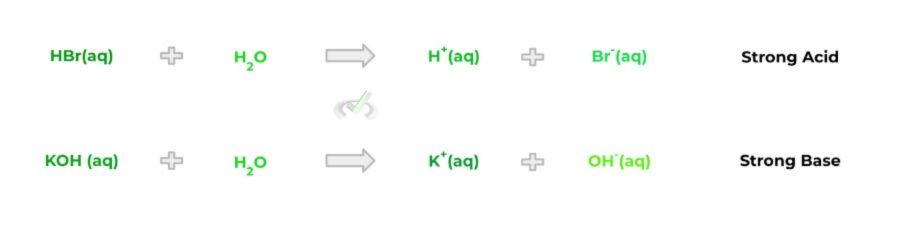
Now compare this to weak acids/bases which, conversely, do not completely dissociate into their respective hydrogen and hydroxide ions. When the weak acids and bases dissociate, the reaction is reversible, as shown by the equations below:
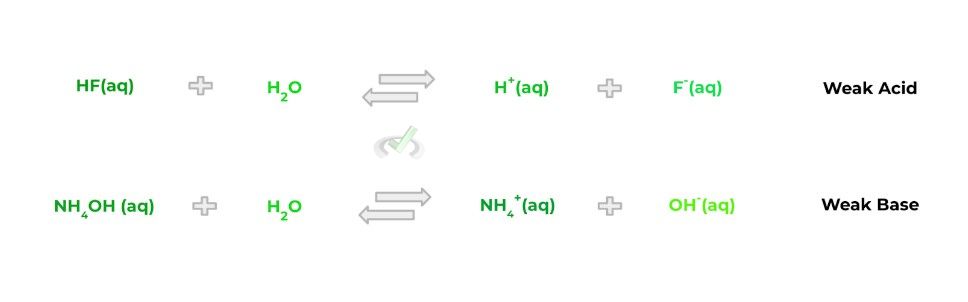
In order to get a better visual understanding of the differences in strength and dissociative properties of strong and weak acids/bases, check out the figure below detailing the 2:
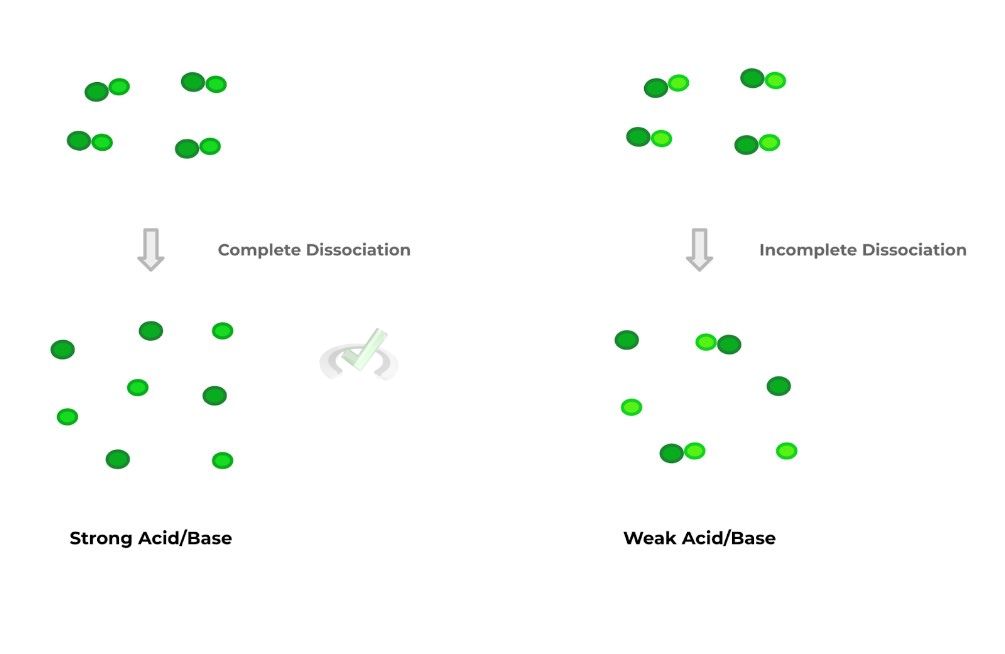
Hopefully the above visual gives you a better idea of differences in dissociation between strong and weak acids. It’s important to note that in reality, both the strong and weak acid/base dissociations are reversible.
However, in regards to strong acids and bases, the equilibrium of the reversible reaction highly favors the products (i.e., the hydrogen ion H+ and cation) that chemists simply view the reaction as irreversible.
Click on this article for a more in depth covering of the differences between strong and weak acids and bases!Full Study Notes : Acid/Base Dissociation and Relation to Acid/Base Strength
For more in-depth content review on the differences between strong and weak acids and bases, check out these detailed lesson notes created by top MCAT scorers.
4. pH and pOH
It’s often helpful to have values that can give us some insight into the strength of an acid/base. In this case, we can use 2 values called pH and pOH, which you may be familiar with if you’ve taken a basic chemistry course or even through daily life!
The values of pH and pOH are actually derived in a logarithmic scale; to be honest, this is all technical mumbo jumbo, and the only thing you need to know is that the value of the pH/pOH will increase by 1 every time the concentration of H+/OH- increases by x10. Take a look at the chart below to get a better feel!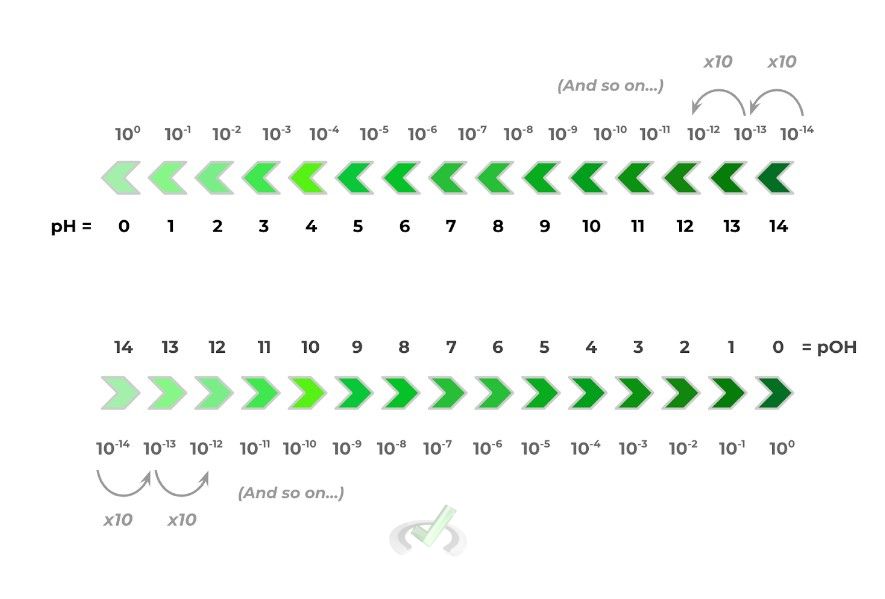
There are a lot of numbers and values to take in, but one of the main ideas is that they’re opposites of one another: as the value of pH increases, the value of pOH decreases. In addition, try to utilize the following trends to your advantage:
a. If the value of pH is smaller/decreasing ⇒ acidity of the solution is increasing!
Why?
This is because the concentration of H+ within the solution is increasing, which is why the solution is becoming more acidic.
Likewise, the inverse relationship between pH and pOH indicates that the pOH value of the solution is increasing because of the smaller OH- concentration.
b. If the value of pH is bigger/increasing ⇒ basicity of the solution is increasing!
Why?
In this case, conversely, the concentration of OH- within the solution is increasing, which is why the solution is becoming more basic.
As such, opposite to the above scenario, the pOH value of the solution is decreasing with the increasing concentration of OH- within the solution!
One equation to become familiar with come test day is shown below, which allows us to solve for the pH of a solution given that we have the concentration of H+ ions in molarity. Note that this can also be used to determine the pOH — all that’s needed is to substitute the concentration of H+ with OH- ions.

Full Study Notes : pH and pOH
For more in-depth content review on the importance of pH and pOH values in determining the acidity/basicity in a solution, check out these detailed lesson notes created by top MCAT scorers.
5. Acid-Base Neutralization
Before getting into the topics of titration and buffers, it’s important to establish a basic understanding of neutralization reactions, as these provide the basis for why titrations and buffers occur the way they do.
Think of a neutralization reaction as a “canceling out” reaction: simply put, acid and base will react with one another to produce salt and water. The salt is composed of the cation and anion, which originate from the base and acid, respectively.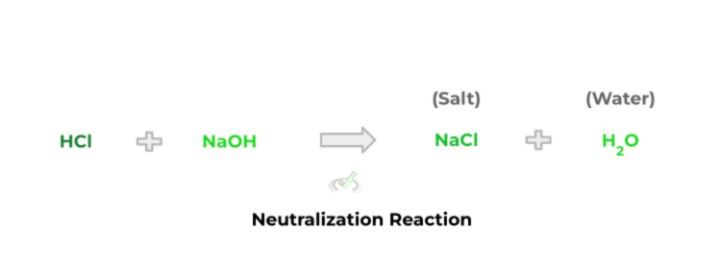
One thing to note about the reaction above is that it’s an example of a double displacement reaction! Recall that this type of reaction occurs when there’s an exchange of ions between 2 reactants.
Notice how the chloride ion of hydrochloric acid is “swapped” with the hydroxide ion of sodium hydroxide in order to form the sodium chloride salt. Additionally, because the ions are swapped, a water molecule is also produced in the reaction.
Learn more about neutralization reactions in this article in order to build the foundation for titrations and buffers!
Full Study Notes : Acid-Base Neutralization
For more in-depth content review on the acid-base neutralization, check out these detailed lesson notes created by top MCAT scorers.
6. Titrations
Having covered the basics of neutralization reactions, we can go ahead and tackle the topic of titrations. Titrations are a strategy we can use in order to determine the unknown concentration of an acid or base. For simplicity of the chapter overview, we’ll focus on “strong acid/strong base” titrations.
In titrations, you’ll be given the volume of the unknown acid/base — this is called the analyte because this is the solution we’re analyzing to find the concentration (in this case, the strong acid).
The other solution we have is the strong base which we know the concentration and volume of. In order to perform a titration, we can slowly add a strong base until all the strong acid has been neutralized.
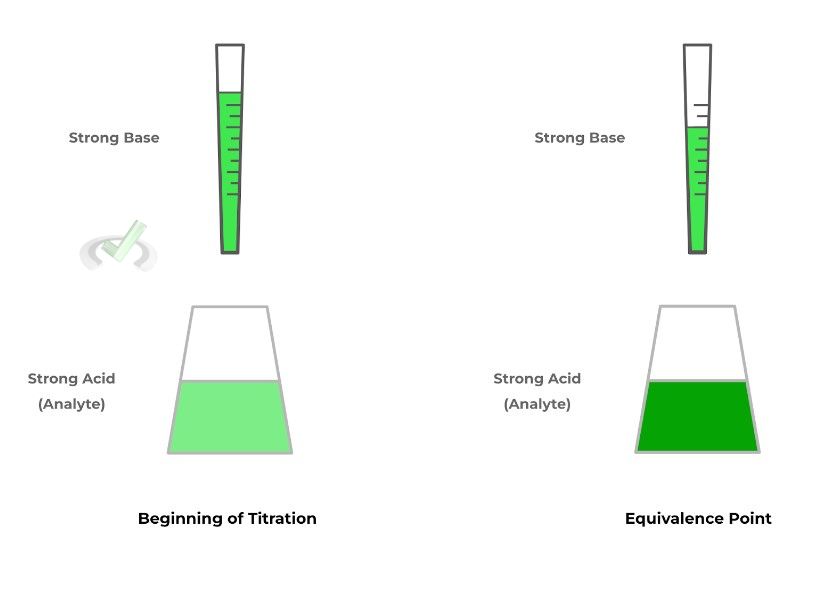
You probably noticed the term equivalence point, which is the point where enough strong base is added in order to completely neutralize the strong acid solution — at this point, the pH of the solution is a neutral 7 because, neither the acid nor base dominates!
From here, we can actually use a derivation of the M1V1 = M2V2 equation in order to determine the molarity of the strong acid! In this case, we set it up the same way, except we replaced the subscripts 1 and 2 with A and B to represent the strong acid and base, respectively.

This set-up should make sense as we’re trying to solve for the molarity of the strong acid, which is our unknown value. We know that the moles of strong acid and base should be equal because at the equivalence point. Complete neutralization has occurred where an equal amount of base has reacted with an equal amount of acid.
Again, it’s important to note that this is only true for strong acid and strong base titrations which are generally considered the simplest. Things get a little more complicated with other types of titrations such as weak acid/strong base or weak base/strong acid which we cover in detail in this article!Full Study Notes : Titrations
For more in-depth content review on titrations, check out these detailed lesson notes created by top MCAT scorers.
7. Buffers
Sometimes, when we want to perform a reaction, we need to run it under a certain pH range in order to attain a specific outcome. This is where the use of buffers is so beneficial, as these are solutions that have the capacity to reduce changes in pH, even with the slight addition of acid and base.
In order to form a buffer, it’s important that we use a weak acid (or base) as well as its corresponding conjugate base (acid) salt. It’s these 2 components that allow for buffers to resist change to pH: any added acid will react with the conjugate base, while any added base will react with the weak acid. Take a look at the example below!
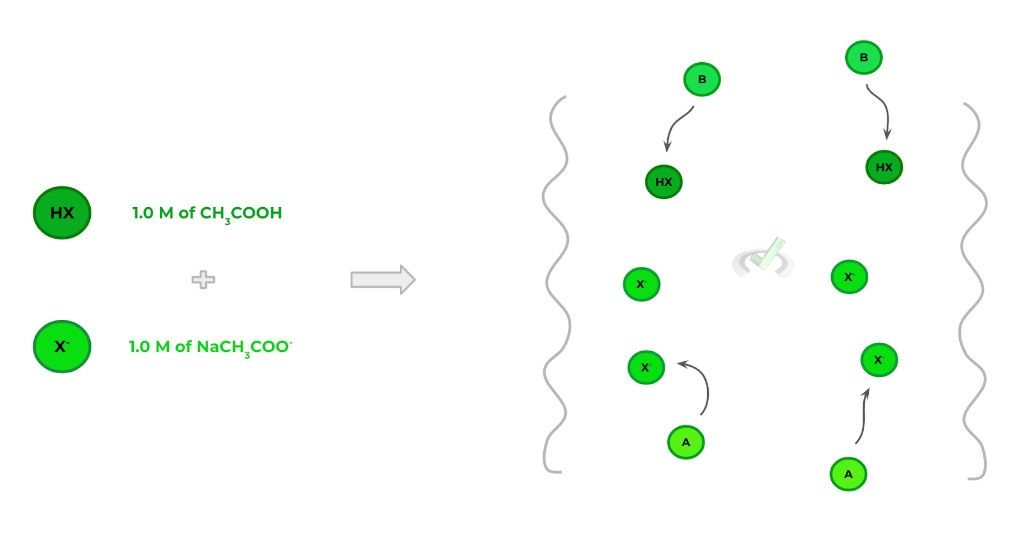
As shown above, we could form a buffer solution with 1.0 M of CH3COOH and 1.0 M NaCH3COO-. Here, HX and X- represent the weak acid and its conjugate base, respectively. Likewise, A and B represent incoming acid and base, respectively.
You may be familiar with a real life application of the buffer system that’s taking place within your body right now! In order for our blood to be within a healthy pH range (~7.35 - 7.45), we use carbonic acid (a weak acid) and its conjugate base counterpart, bicarbonate, in order to maintain our blood pH balance!
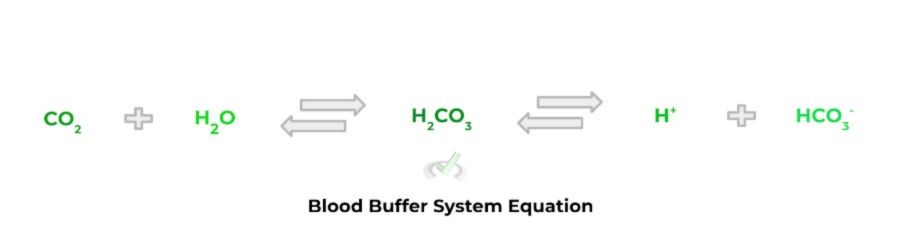
Full Study Notes : Buffers
For more in-depth content review on buffers and the amazing blood buffer system, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about acids and bases!
Term | Definition |
|---|---|
Arrhenius Acid/Base | One definition of acid and bases; Defined as a species that dissociates into a H+ or OH- ion, respectively |
Bronsted-Lowry Acid/Base | One definition of acid and bases; Defined as a species that donates a proton or accepts a proton, respectively |
Lewis Acid/Base | One definition of acids and bases; Defined as a species that accepts an electron pair or donates an electron pair, respectively |
Strong Acid/Base | An acid or base which dissociates completely; The dissociation reaction is best thought of as irreversible |
Weak Acid/Base | An acid or base which dissociates incompletely; The dissociation reaction is best thought of as reversible |
pH | A logarithmic value which gives an indication of the acidity of a solution; The lower a pH value is, the more acidic the solution is |
pOH | A logarithmic value which gives an indication of the basicity of a solution; The lower a pOH value is, the more basic the solution is |
Neutralization Reaction | A double displacement reaction between an acid and base which results in the formation of water and a salt |
Titration |
A laboratory technique utilized to determine the concentration of an acid or base (called the analyte) |
Buffer |
A solution composed of a weak acid/base and its corresponding conjugate base/acid, usually in a salt form |
Blood Buffer System |
A buffer system utilized within the blood which uses carbonic acid and bicarbonate in order to maintain a narrow range of blood pH (~7.35 - 7.45) |
Additional FAQs - Acids and Bases on the MCAT
A. How Do You Remember Strong Acids and Bases on the MCAT?
In addition, in this case of oxyacids, acids that end in the suffix “-ic” will generally be strong! Consider the difference between nitric and nitrous acid: the former is a strong acid, while the latter is a weak acid.
B. Do You Need to Know Strong Acids and Bases – MCAT?
Additionally, be sure to also be aware of the main characteristics of strong acids and bases, such as complete dissociation, a very high pH when dissolved in solution, and any other main concepts!
C. How Do You Remember Acids and Bases – MCAT?
Additionally, when trying to remember the differences in their characteristics, just know that acids and bases are the opposite of one another! By memorizing the characteristics of one, you already know the characteristics of the other, as it’s just the opposite!
D. What is a Buffer – MCAT?
Additional Reading Links – Study Notes for Acid and Bases on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
