Though some of us here at MedLife Mastery are big fans of the Avatar: The Last Airbender series, it’s with disappointment to say that the 4 elements of water, earth, fire, and air aren't completely scientifically accurate — sighhh.
In reality, there are currently 118 known elements as stated by the American Chemical Society which are all neatly organized in the periodic table — it’s with further disappointment to say that we can’t bend these elements like in the show.
However, we’ll still go ahead and cover how elements can be grouped and the important trends we see on the periodic table. Let’s get started!
Periodic Table on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
In regards to questions covering periodic table organization and trends, try to expect around 4-6 question on average covering these topics!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Periodic Table
If you can, try to brush up a little on the general atomic structure of atoms, specifically the organization of electrons into subshells! This will be important when covering the rationale behind some of the periodic table trends that are commonly observed!
Probably similar to your other General Chemistry college courses, you won’t be expected to memorize the elements of the periodic table in regards to their atomic number, name, etc. The MCAT will provide you with a table for the test, giving you one less thing to review for the exam.
1. Periodic Table Basics and Main Elemental Categories
While it’s definitely good to be familiar with some of the elements of the periodic table, it’s much more important to understand the main breakdowns and elemental arrangements on the periodic table as the way elements are organized is reflective of their similar properties and characteristics.
One way the periodic table can be broken down is via rows and groups. Rows (also called periods) move left to right across the periodic table; conversely, groups (also called columns) move top to bottom down the periodic table. Check out the example below!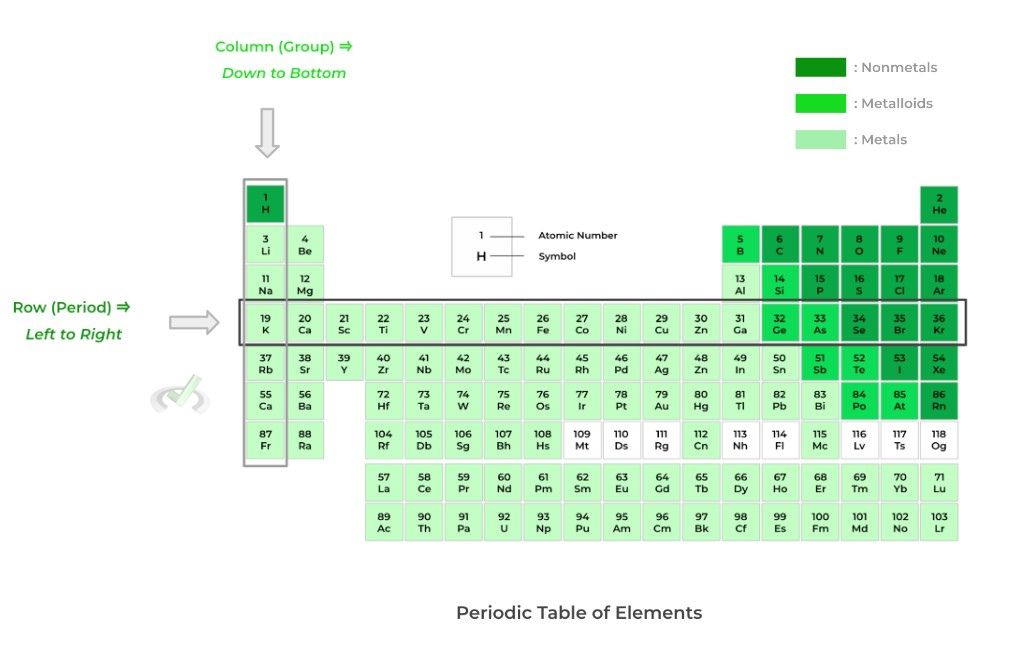
As you move left to right across the period — passing each element one by one — you add one proton and one electron. Moving down in a column, the principal quantum number (n) increases by one!
Finally, another important note to make is that elements within the same column have the same electronic configuration in their valence (outermost) shell! This often results in them having very similar chemical characteristics and reactivity!
As shown above, there are 3 main elemental categorizations for the elements on the periodic table: 1) metals, 2) nonmetals, and 3) metalloids. Let’s give a brief listing of some of their shared properties and characteristics!
A. Metals
B. Nonmetals
C. Metalloids
Full Study Notes : Periodic Table Basics and Main Elemental Categories
For more in-depth content review on the basic elemental categorizations and organization of the periodic table, check out these detailed lesson notes created by top MCAT scorers.
2. Importance of Valence Electrons and Effective Nuclear Charge
Before getting into the latter part of the title above, let’s quickly review valence electrons! Recall that valence electrons are the electrons located in the outermost shell of an atom! As a result, these are the electrons that usually dictate the reactivity of the element!
Effective nuclear charge (Zeff) refers to the inward, “pulling” force of the positive nuclear charge on the valence electrons — here, the positive charge of protons which attracts the negatively charged valence electrons! Check out the equation below!
It’s important to note that the magnitude of the positive nuclear force on the valence electrons is affected by the inner electrons, as noted in the equation.
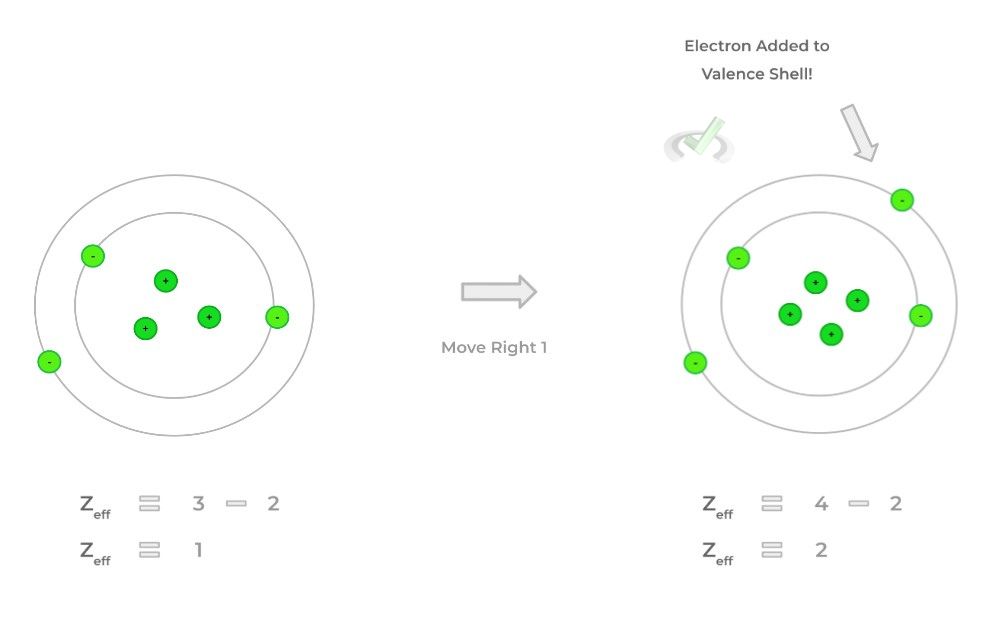
This is due to the effect called electron shielding — this refers to how the inner electrons “shield” the positive attraction of the nuclear protons. Let’s go into some key trends below!A. Left to Right Across a Row: Zeff Increases
>> Why?
- When moving across a row, electrons are added to the valence (outermost) shell while the protons are also added. In this case, the number of inner core electrons remains the same!
- This results in Zeff increasing moving across the table because the “Z” value is increasing while “S” remains constant!
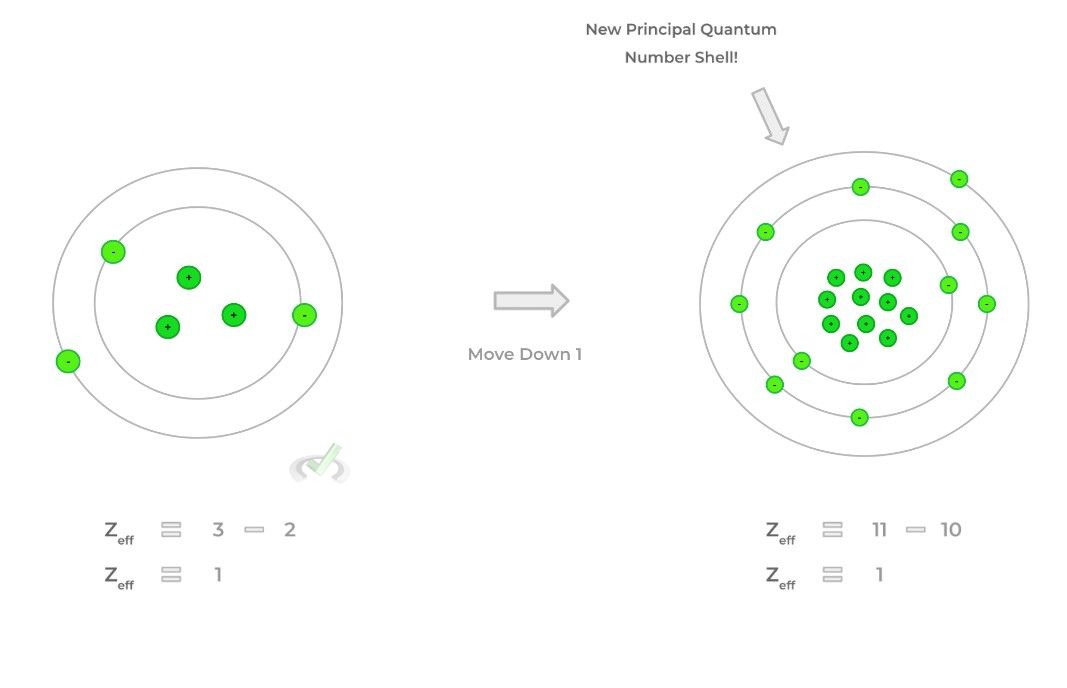
B. Up to Bottom Down a Column: Zeff Remains Constant
>> Why?
- When moving down a column, the principal quantum number increases by 1! As such, you’ve accumulated all the inner electrons from the previous column which can be used in electron shielding!
- Even though the number of protons increases, the amount of electrons that can be used for electron shielding equally cancels out the positive charge and keeps the effective nuclear charge relatively constant.
Full Study Notes : Importance of Valence Electrons and Effective Nuclear Charge
For more in-depth content review on importance of valence electrons and effective nuclear charge, check out these detailed lesson notes created by top MCAT scorers.
3. Trends of the Periodic Table
A great benefit to how elements are organized in the periodic table is that, for the most part, elements closer to one another on the periodic table tend to have similar properties and characteristics, as we mentioned before!
From these comes various periodic table trends of various elemental properties! In this section, we’ll cover some of the high yield trends of the periodic table as well as the rationale behind why these trends occur. We’ll primarily cover trends in atomic radius, electron affinity/negativity, and ionization energy!
While of course you can memorize the periodic table trends, you can also memorize the rationale if that’s more beneficial to you! Instead of memorizing, you’ll actually work your way through the problem.
A. Atomic Radius: Top to Bottom -- Increases; Left to Right -- Decreases
>> Why?
Going down a column, the principal quantum number shell increases by one, which distances the valence shell more and increases the atomic radius!
When moving to the right across a row, recall that the Zeff increases because the number of inner core electrons remains constant while the number of protons increases!
This results in a greater positive “inward, pulling” magnitude from Zeff on the valence electrons, which decreases the atomic radius!
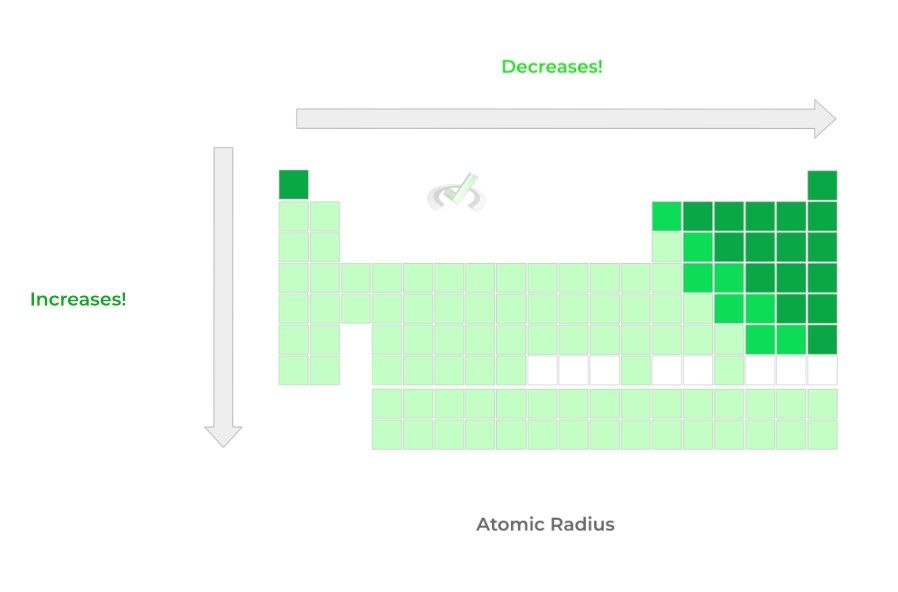
**Note: The fully outlined periodic table with all the elements was not included for visual simplicity!
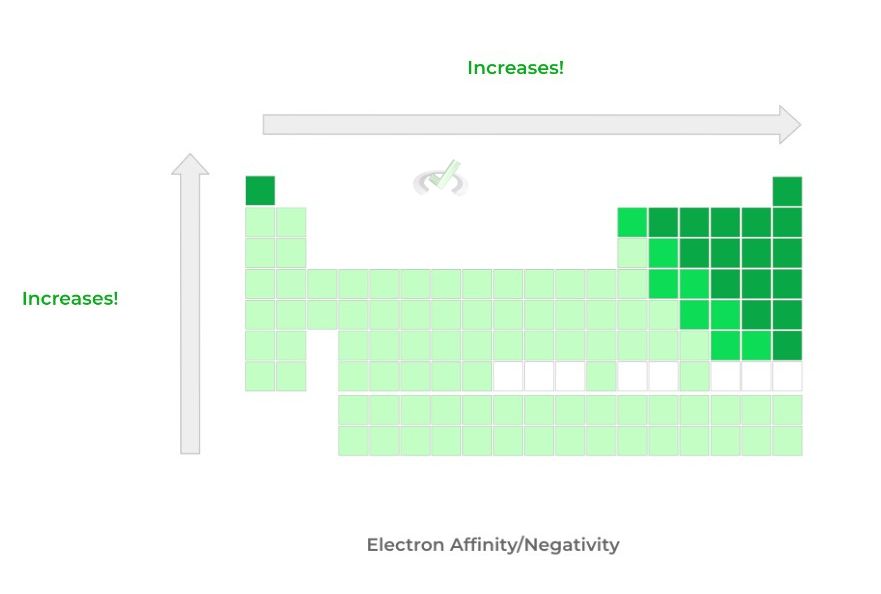
B. Electron Affinity/Negativity: Bottom to Top -- Increases; Left to Right -- Increases
>> Why?
A great way to think about these trends is to look at the valence electrons of the elements! Elements to the left of the table, such as those in group 1A, have 1 electron in their valence shell.
As such, these elements are looking to lose electrons rather than gain in order to complete their octet.
Elements on the right side of the table, such as those in group 7, have 7 electrons in their valence shell -- as such, it’s much easier to complete the octet by adding an electron!
It’s important to note that noble gasses, the right-most group, are excluded from this trend because these gasses already have a complete octet!
Likewise, as we move down a column, the principal quantum number shell increases, which distances the positive nuclear attraction for electrons. As such, electron affinity/negativity decreases.
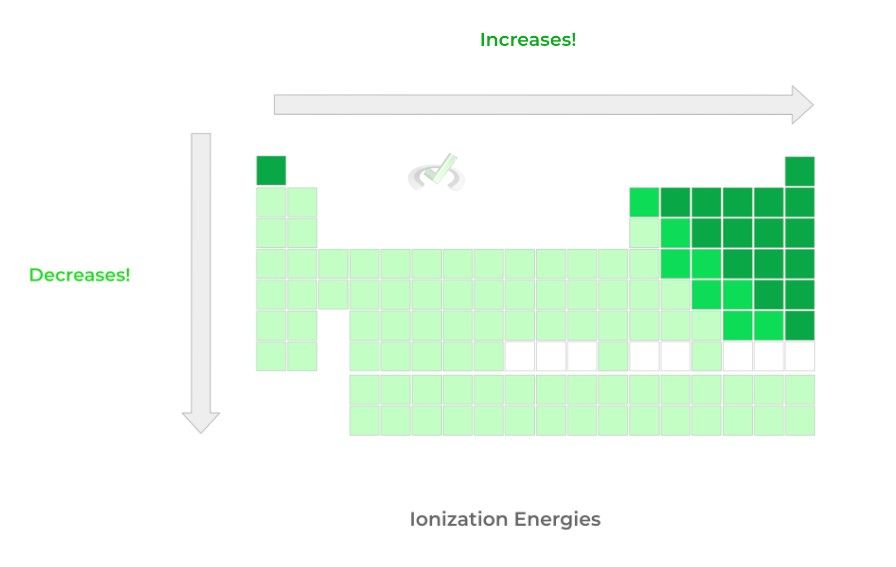
C. Ionization Energy: Top to Bottom -- Decreases; Left to Right -- Increases
>> Why?
Luckily, ionization energies follow the same trends and rationale as electron affinity/negativity! Those on the left side want to lose electrons to complete their octet (i.e. low IE) while those on the right want to gain electrons (i.e. high IE) to complete the octet!
Similarly, because the principal quantum number shell is increased going down, the valence shell is distanced from the positive, nuclear attraction inwards, resulting in lower IEs!
Full Study Notes : Trends of the Periodic Table
For more in-depth content review on main trends of the periodic table, check out these detailed lesson notes created by top MCAT scorers.
4. Specific Chemical Groups
Let’s go ahead and talk about some of the specific chemical groups present in the periodic table! Because we’re talking about chemical groups (columns), it’s best to discuss these chemical groups in reference to their valence electrons!
A. Alkali and Alkali Earth Metals (Group 1 and 2)
Being towards the left side of the periodic table, these elements have low electron affinity/negativity and ionization energies! The latter is due to the fact that these metals have only 1 and 2 electrons in their valence shells!
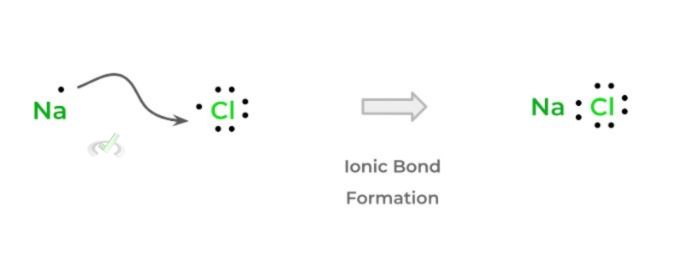
This makes them very prone to form cations, as it’s much easier to lose the electrons from their valence shells in order to form a complete octet! Being cations, these elements tend to form ionic bonds with other anionic species!

B. Chalcogens and Halogens (Groups 16 and 17)
Conversely, these elements tend to have high electron affinity/negativity and ionization energies being at the right side of the periodic table. Because they have 6 and 7 electrons in their valence shells, it’s much more feasible for them to add electrons to complete their octet.
In addition, you’ll often see these elements form ionic compounds with the alkali and alkali earth metals due to a transfer of electrons!
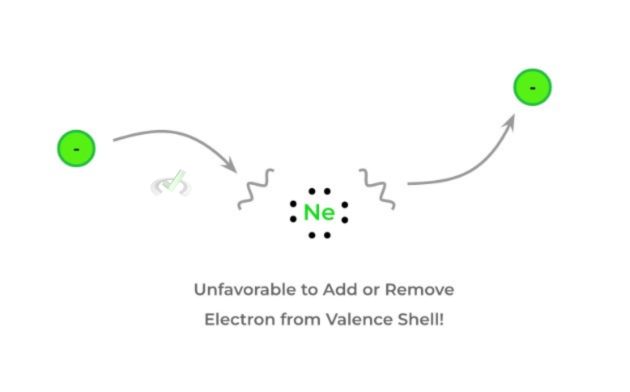
C. Noble Gasses (Group 18)
Noble gases are a unique group of elements because they are characterized by their unreactivity! This is due to the fact that there’s already a full octet in their valence shell, which makes them not as likely to add or give up electrons!
D. Transition Metals (Groups 3 to 12)
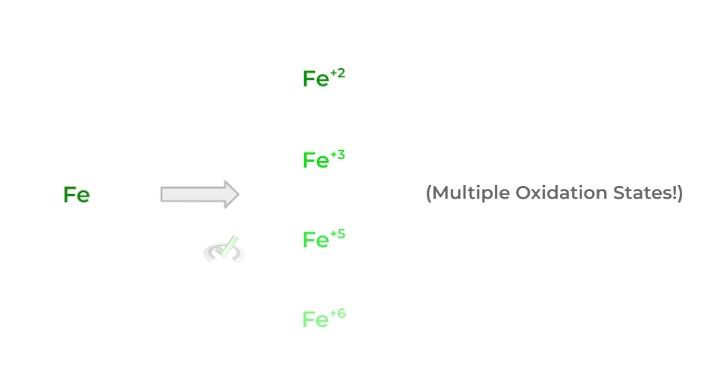
These metals are often called the “d-block” elements because the highest energy subshell in their electronic configuration is a d subshell. Transition metals are also known to have relatively low ionization energies and electron affinity/negativity.
This results in them adopting many different oxidation states — this is why oftentimes transition metals are included as a component of many reducing agents!
Full Study Notes : Specific Chemical Groups
For more in-depth content review on specifics about the different chemistry of different chemical groups, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about the periodic table in general chemistry!
Term | Definition |
|---|---|
Row | Also called period; Refers to moving left to right across a single row |
Column | Also called group; Refer to moving top to bottom down a single column |
Metals | One of the 3 main elemental categorizations; Characterized by their malleability, conductivity, and low ionization energies and electron affinities/negativities |
Nonmetal | One of the 3 main elemental categorizations; Characterized by having opposing characteristics to those found in metals such as high ionization energies and electron affinities/negativities |
Metalloids | One of the 3 main elemental categorizations; Characterized by having similar properties to both metals and nonmetals |
Effective Nuclear Charge | The magnitude of the positive, inward pulling attraction from the nuclear protons on the valence electrons |
Valence Electrons | Electrons which are found on the outermost shell of an elemental atom |
Electron Shielding | The effect where inner, core electrons (those not in valence) shell reduce the effective nuclear charge felt on the valence shell electrons |
Electron Affinity | Refers to the attraction a nucleus has on an incoming electron |
Electronegativity | Refers to the attraction a nucleus has one electrons within a covalent bond |
Ionization Energies |
Refers to the energy required to release an electron from the valence shell |
Alkali and Alkali Earth Metals |
Found in groups 1 and 2 and have one and two valence electrons, respectively; Prone to form cations in order to fulfill octet rule |
Chalcogens and Halogens |
Found in groups 16 and 17 and have six and seven valence electrons, respectively; Tend to gain electrons to form anions |
Noble Gasses |
Found in group 18 and have 18 valence electrons; Generally unreactive because of their complete octet in the valence shell |
Transition Metals |
Found in groups 3-12 and are characterized by their ability to attain multiple oxidation states due to their relatively low ionization energies |
Additional FAQs - Periodic Table on the MCAT
Is the Periodic Table Given on the MCAT?
How do I Remember the Periodic Table? – MCAT
Are Orbitals on the MCAT?
What is Electron Affinity? – MCAT
It's similar to electronegativity, except electronegativity refers to the electron attraction of an atom within a covalent bond. For electron affinity, the element is neutral and free, not attached to a bond!
Additional Reading Links – Study Notes for Periodic Table on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Acids and Bases on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
