The Common Ion Effect is an important concept in general chemistry that affects the solubility of salts and the behavior of acids and bases. Understanding this effect is crucial for predicting and controlling chemical reactions in various environments, including industrial processes and biological systems.
I. Introduction to the Common Ion Effect
The Common Ion Effect describes how adding a common ion to a solution affects the solubility equilibrium of an ionic compound. Let's break down this concept with clear definitions and examples.
A. Definition and Basic Principle
The Common Ion Effect occurs when a solution contains two sources of the same ion. This added common ion reduces the solubility of the ionic compound already in the solution. For example, if we add sodium chloride (NaCl) to a solution containing silver chloride (AgCl), the solubility of AgCl decreases because both NaCl and AgCl release chloride ions (Cl⁻) into the solution.
B. Understanding Solubility Equilibrium
Solubility equilibrium is the balance between a dissolved ion and its undissolved solid form. The equilibrium can be represented as:
AgCl (s) ⇌ Ag+ (aq) + Cl- (aq)
When a common ion (Cl⁻ in this case) is added, the equilibrium shifts to the left, meaning more AgCl remains solid and less is dissolved in the solution. This follows Le Chatelier's Principle, which states that a system at equilibrium will adjust to counteract any changes.
II. Practical Applications of the Common Ion Effect
The Common Ion Effect has many practical applications, especially in precipitation reactions and buffer solutions. Understanding these applications helps in predicting and controlling chemical reactions.
A. Precipitation Reactions
To remove lead ions (Pb²⁺) from water, we can add a solution of sodium sulfate (Na₂SO₄). The sulfate ions (SO₄²⁻) will combine with lead ions to form lead sulfate (PbSO₄), a poorly soluble salt:
Example: Removal of Lead Ions
Pb2+ (aq) + SO42- (aq) ⇌ PbSO4 (s)
Adding more sulfate ions from Na₂SO₄ shifts the equilibrium to the right, precipitating PbSO₄ and effectively removing lead ions from the water.
B. Buffer Solutions
A buffer solution of acetic acid (CH₃COOH) and sodium acetate (CH₃COONa) maintains pH through the Common Ion Effect. When an acid (H⁺) is added, acetate ions (CH₃COO⁻) from sodium acetate react with the hydrogen ions to form more acetic acid:
Example: Acetic Acid and Sodium Acetate Buffer
CH3COO- (aq) + H+ (aq) ⇌ CH3COOH (aq)
Conversely, when a base (OH⁻) is added, the acetic acid donates hydrogen ions to neutralize the hydroxide ions, forming water and acetate ions.
III. Calculations Involving the Common Ion Effect
Understanding how to calculate the changes in solubility due to the Common Ion Effect is crucial for predicting chemical reaction outcomes. These calculations often involve the solubility product constant (Ksp).
A. Solubility Product Constant (Ksp)
The solubility product constant (Ksp) measures a salt's solubility. It is the product of the ions' molar concentrations, each raised to the power of their coefficients in the balanced equation.
For AgCl:
Ksp = [Ag+][Cl-]
B. Example Calculation
Consider a solution where we add NaCl to a saturated solution of AgCl. The initial concentration of Cl⁻ from AgCl equals the solubility of AgCl (s). When NaCl is added, it provides additional Cl⁻ ions.
If the initial solubility of AgCl (s) is 1.3 x 10⁻⁵ M, and we add 0.01 M NaCl, the new concentration of Cl⁻ ions will be:
[Cl-] = 1.3 x 10-5 M + 0.01 M ≈ 0.01 M
Using the Ksp value for AgCl (1.8 x 10⁻¹⁰):
1.8 x 10-10 = [Ag+][0.01 M]
Solving for [Ag⁺]:
The solubility of Ag⁺ decreases significantly due to the Common Ion Effect.
IV. Illustrations and Equations
Visual aids can enhance the understanding of the Common Ion Effect. Here are some key chemical equations and suggested illustrations.
A. Chemical Equations
Here are some key chemical equations illustrating the Common Ion Effect:
1. AgCl Solubility in Water:
AgCl (s) ⇌ Ag+ (aq) + Cl- (aq)
2. Effect of Adding NaCl:
AgCl (s) ⇌ Ag+ (aq) + Cl- (aq) (from NaCl)
3. Precipitation of PbSO4:
Pb2+ (aq) + SO42- (aq) ⇌ PbSO4 (s)
V. Bridge/Overlap
The Common Ion Effect is not just limited to solubility. It connects to several broader chemistry concepts that are important for the MCAT.
A. Le Chatelier's Principle
The Common Ion Effect is a specific application of Le Chatelier's Principle. This principle states that a system at equilibrium will adjust to counteract any changes. Understanding this principle is foundational in many areas of chemistry.
B. Acid-Base Equilibria
The behavior of buffer solutions under the Common Ion Effect is closely related to acid-base equilibria. Understanding this connection helps predict the outcomes of adding acids or bases to buffer systems, which is especially important for the MCAT.
C. Solubility and Precipitation
The Common Ion Effect plays a significant role in controlling the solubility and precipitation of salts. This is crucial in various fields, from environmental science to pharmaceuticals. Knowing how to manipulate solubility can help design better drugs and clean up pollutants.
D. Biological Systems
The Common Ion Effect is also important in biological systems. For example, it helps maintain the pH balance in blood and other bodily fluids. This concept is tested on the MCAT about biochemical pathways and physiological processes.
VI. Wrap-Up and Key Terms
The Common Ion Effect is a fundamental concept in chemistry. It helps us understand how a common ion's presence can influence compounds' solubility. This knowledge is essential for predicting and controlling chemical reactions in various contexts, including industrial processes, environmental science, and biological systems.
Key Terms
VII. Practice Questions
Sample Practice Question 1
What happens to the solubility of calcium sulfate (CaSO₄) in water if calcium chloride (CaCl₂) is added?
A. Increases
B. Decreases
C. Stays the same
D. Becomes zero
Ans. B
Adding CaCl₂ increases the concentration of Ca²⁺ ions in the solution, shifting the equilibrium and decreasing the solubility of CaSO₄.
Sample Practice Question 2
Which of the following best describes the Common Ion Effect?
A. An increase in solubility due to a common ion
B. A decrease in solubility due to a common ion
C. No effect on solubility
D. Complete dissolution of the ionic compound
Ans. B
The Common Ion Effect decreases an ionic compound's solubility when a common ion is added to the solution.


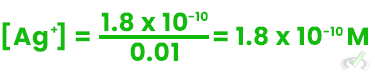





 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
