In chemistry, reactions don’t just stop once they’ve started—they often reach a state where everything is in balance. This state is called equilibrium, where the reactions seem to pause, but there’s more happening beneath the surface. Understanding how this balance works and what happens when it's disturbed is key to mastering the concept of equilibrium in solutions.
I. Introduction to Equilibrium
Equilibrium refers to a state in which the concentrations of reactants and products remain constant over time. It occurs when the forward reaction rate equals the reverse reaction rate.
II. Dynamic Nature of Equilibrium
In a chemical equilibrium, reactions are dynamic, meaning they continue to occur without a net change in the concentrations of reactants and products.
Example:
A + B ⇌ C + D
At equilibrium, the rate of A + B → C + D equals the rate of C + D → A + B.
III. The Equilibrium Constant (K)
The equilibrium constant (K) is a number that expresses the relationship between the concentrations of reactants and products at equilibrium for a given reaction.
General Form:
IV. Le Chatelier’s Principle
Le Chatelier’s Principle states that if a system at equilibrium is disturbed, it will adjust to counteract the disturbance and restore a new equilibrium.
Types of Disturbances
Example:
For the reaction N2 + 3H2 ⇌ 2NH3:
increasing temperature shifts the equilibrium to the left (producing less NH3) because the reaction is exothermic (releases heat).
V. Calculating Equilibrium Concentrations
To calculate equilibrium concentrations, we often organize data and solve for unknowns using an ICE table (Initial, Change, Equilibrium).
Example Problem:
For the reaction N2 + 3H2 ⇌ 2NH3 with Kc = 0.500 at a certain temperature:
- Set up the ICE table with initial concentrations.
- Write the expression for the equilibrium constant.
- Solve for the unknown concentrations at equilibrium.
Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
N2 | 1.0 | -x | 1.0 - x |
H2 | 1.5 | -3x | 1.5 - 3x |
NH3 | 0.0 | +2x | 2x |
VI. Acid-Base Equilibria
Acids and Bases in solutions reach equilibrium based on their strength, represented by their dissociation constants Ka for acids and Kb for bases.
Example:
For acetic acid (CH₃COOH) in water:
VII. Solubility Equilibria
Solubility Equilibria involve the dissolution of solids in water to form a saturated solution.
Example:
For calcium fluoride (CaF₂):
VIII. Common Ion Effect
The common ion effect occurs when a salt containing an ion in common with a dissolved substance is added to the solution, reducing the solubility of the dissolved substance.
Example: Adding NaF to a solution of CaF₂ decreases the solubility of CaF₂ because the concentration of F⁻ increases, shifting the equilibrium.
IX. Bridge/Overlap
Understanding equilibrium helps in other areas of chemistry, such as:
Reaction Kinetics
Equilibrium concepts explain how fast reactions reach equilibrium and how catalysts affect these rates.
Thermodynamics
The position of equilibrium relates to the energy changes in reactions. Understanding Gibbs free energy helps predict the direction of equilibrium.
Biochemistry
Equilibrium principles apply to metabolic pathways and enzyme reactions. For example, in glycolysis, the equilibrium of each step ensures efficient energy production.
X. Wrap-Up and Key Terms
Understanding equilibrium in solutions involves mastering several key concepts and terms. Equilibrium explains how reactions reach a state where reactants and products do not change over time. This balance is influenced by concentration, temperature, and pressure changes.
Key Terms
XI. Practice Questions
Sample Practice Question 1
What happens to the equilibrium position if the concentration of a product is increased?
A. The equilibrium shifts to the right.
B. The equilibrium shifts to the left.
C. The equilibrium remains unchanged.
D. The reaction stops.
Ans. B
Increasing product concentration shifts equilibrium left to form more reactants, balancing the system by reducing excess product and restoring equilibrium.
Sample Practice Question 1
How does decreasing the temperature affect the equilibrium of an exothermic reaction?
A. The equilibrium shifts to the right, favoring product formation.
B. The equilibrium shifts to the left, favoring reactant formation.
C. The equilibrium remains unchanged.
D. The reaction rate increases.
Ans. A
Decreasing temperature in an exothermic reaction shifts equilibrium right, favoring product formation. The system compensates by producing more heat to counter the temperature drop.



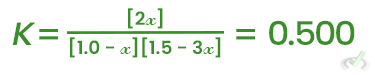
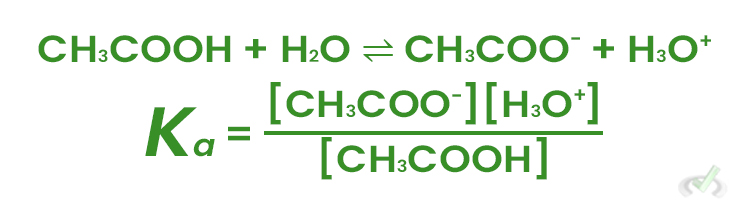
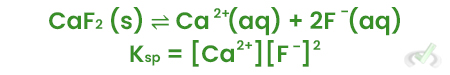





 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
