Understanding the differences between ideal and real gases is crucial in chemistry. This knowledge helps predict gas behavior under different conditions, essential in various scientific and industrial applications.
I. Introduction to Gas Laws
Gases behave differently under varying conditions of temperature and pressure. The study of gases involves understanding the laws that govern their behavior. Let's start by defining ideal and real gases.
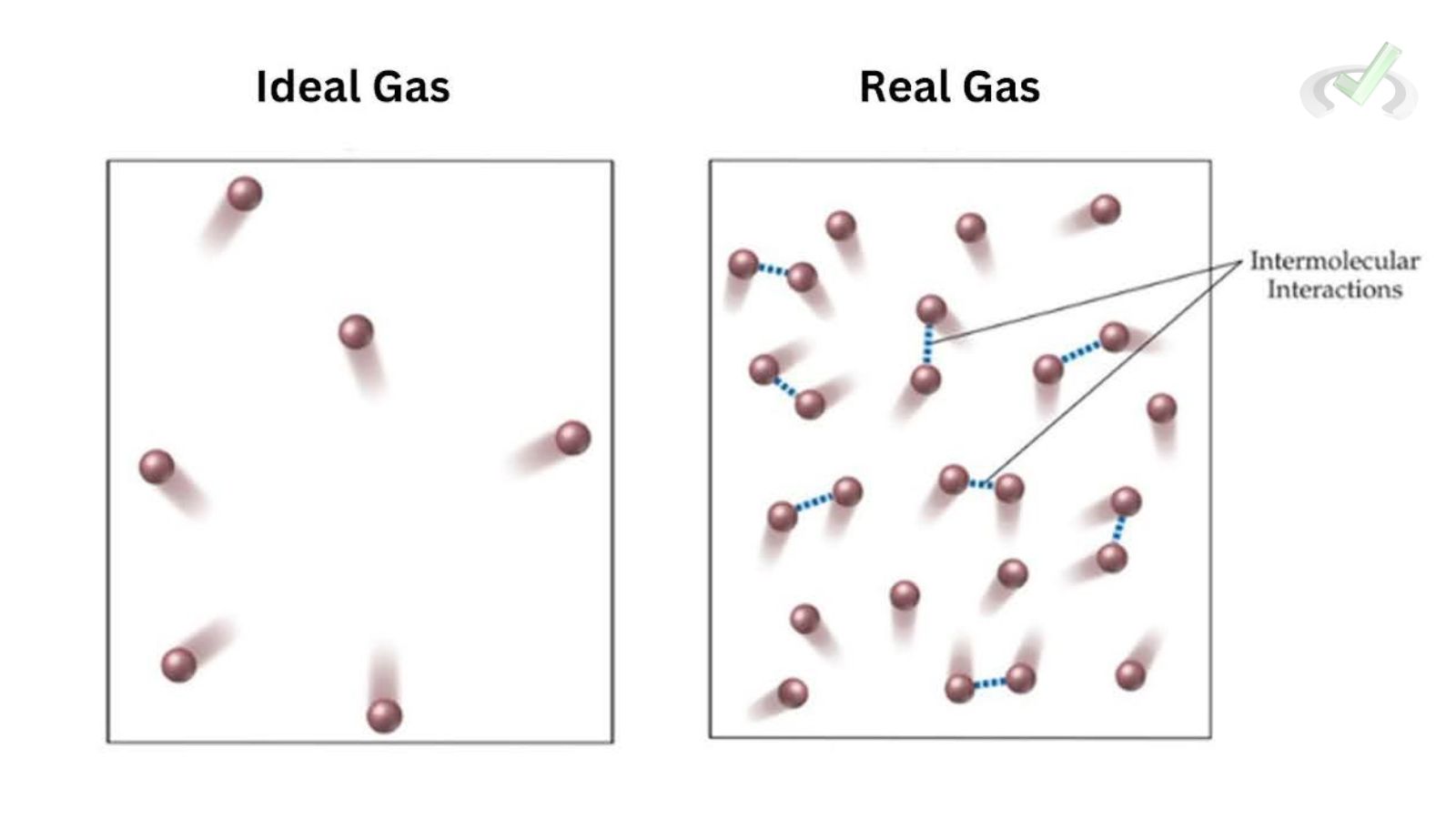
Ideal Gas
An ideal gas is a theoretical gas composed of randomly moving, non-interacting point particles. These particles are considered to have no volume and no intermolecular forces. The Ideal Gas Law describes the behavior of ideal gases:
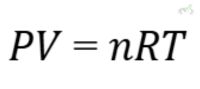
Where:
- P is pressure,
- V is volume,
- n is the number of moles,
- R is the ideal gas constant,
- T is temperature.
Real Gas
A real gas deviates from the ideal gas behavior at high pressures and low temperatures due to intermolecular forces and the finite size of gas molecules. The Van der Waals equation better describes the behavior of real gases:
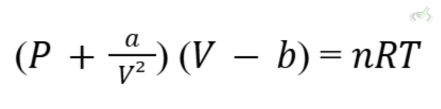
Where:
- a and b are constants specific to each gas. They represent intermolecular attractions and the volume occupied by gas molecules, respectively.
II. Ideal Gas Law Explained
The Ideal Gas Law combines several fundamental gas laws. Here's a breakdown:
Boyle's Law
Boyle's Law states that the volume of a gas is inversely proportional to its pressure at constant temperature:
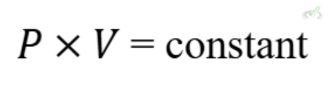
Example: If you compress a balloon, decreasing its volume, the pressure inside the balloon increases.
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature at constant pressure:
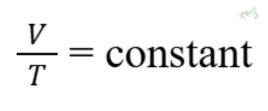
Example: When a balloon is heated, it expands because the gas molecules move faster and push it outward.
Avogadro's Law
Avogadro's Law states that the volume of a gas is directly proportional to the number of moles at constant temperature and pressure:
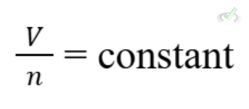
Example: Adding more gas to a balloon increases its volume if the temperature and pressure are constant.
These laws combine to form the Ideal Gas Law.
III. Deviations from Ideal Behavior
Real gases deviate from ideal behavior due to two main factors:
Intermolecular Forces
At high pressures and low temperatures, gas molecules are close together, and intermolecular forces become significant. These forces can attract or repel molecules, causing deviations from ideal behavior.
Types of Intermolecular Forces:
- Van der Waals forces: Weak attractions between molecules.
- Hydrogen bonding: Stronger than Van der Waals forces, occurring between molecules with hydrogen attached to electronegative atoms like oxygen or nitrogen.
Finite Volume of Gas Molecules
Gas molecules occupy a finite volume. At high pressures, this volume becomes significant compared to the gas's total volume, leading to deviations from ideal behavior.
IV. Van der Waals Equation
The Van der Waals equation adjusts the Ideal Gas Law to account for the deviations observed in real gases. It introduces two constants, a and b, to correct for intermolecular forces and molecular volume:
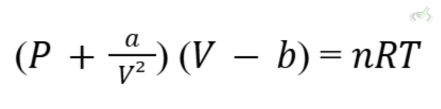
Understanding the Constants:
V. Comparing Ideal and Real Gases
Ideal Gas Behavior
Real Gas Behavior
VI. Applications and Examples
Industrial Applications
Understanding gas behavior is crucial in industrial processes. For instance, the liquefaction of gases like oxygen and nitrogen involves manipulating pressure and temperature to exploit deviations from ideal behavior.
Example: In the Haber process for ammonia production, understanding real gas behavior helps optimize conditions to increase yield.
Environmental Science
Studying atmospheric gases requires knowledge of real gas behavior, especially under varying environmental conditions.
Example: Understanding how greenhouse gases like carbon dioxide and methane behave under different atmospheric conditions is essential for climate modeling.
VIII. Key Terms Review
- Ideal Gas: A theoretical gas with no intermolecular forces and molecules occupying no volume.
- Real Gas: A gas deviates from ideal behavior due to intermolecular forces and finite molecular volume.
- Intermolecular Forces: Forces of attraction or repulsion between molecules.
- Van der Waals Equation: An equation that accounts for the behavior of real gases.
- Compressibility Factor: A measure of how much a gas deviates from ideal behavior.
- Critical Temperature: The temperature above which a gas cannot be liquefied, regardless of pressure.
IX. Practice Questions
Sample Practice Question 1
What happens to the behavior of a real gas as pressure increases?
A. It behaves more ideally.
B. It deviates more from ideal behavior.
C. It remains unchanged.
D. It behaves less ideally.
Ans. B
As pressure increases, intermolecular forces and the finite volume of molecules cause real gases to deviate from ideal behavior.
Sample Practice Question 2
What adjustments does the Van der Waals equation make for real gases?
A. Corrects for temperature.
B. Corrects for pressure.
C. Corrects for volume.
D. Corrects for intermolecular forces and molecular volume.
Ans. D
The Van der Waals equation introduces constants a and b to account for intermolecular forces and the finite volume of gas molecules.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
