Isomers are compounds with the similar molecular formula. However, isomers have different structures or spatial arrangements.
Understanding this concept is fundamental to mastering organic chemistry. Despite having the same number of atoms of each element, they can exhibit different chemical and physical properties.
I. Basics of Isomers
A. What are Isomers?
As mentioned, Isomers are what we call the molecules with the same molecular formula but arranged differently. This difference can be in the atoms' order or spatial orientation. These variations can result in significantly different properties.
B. Types of Isomers
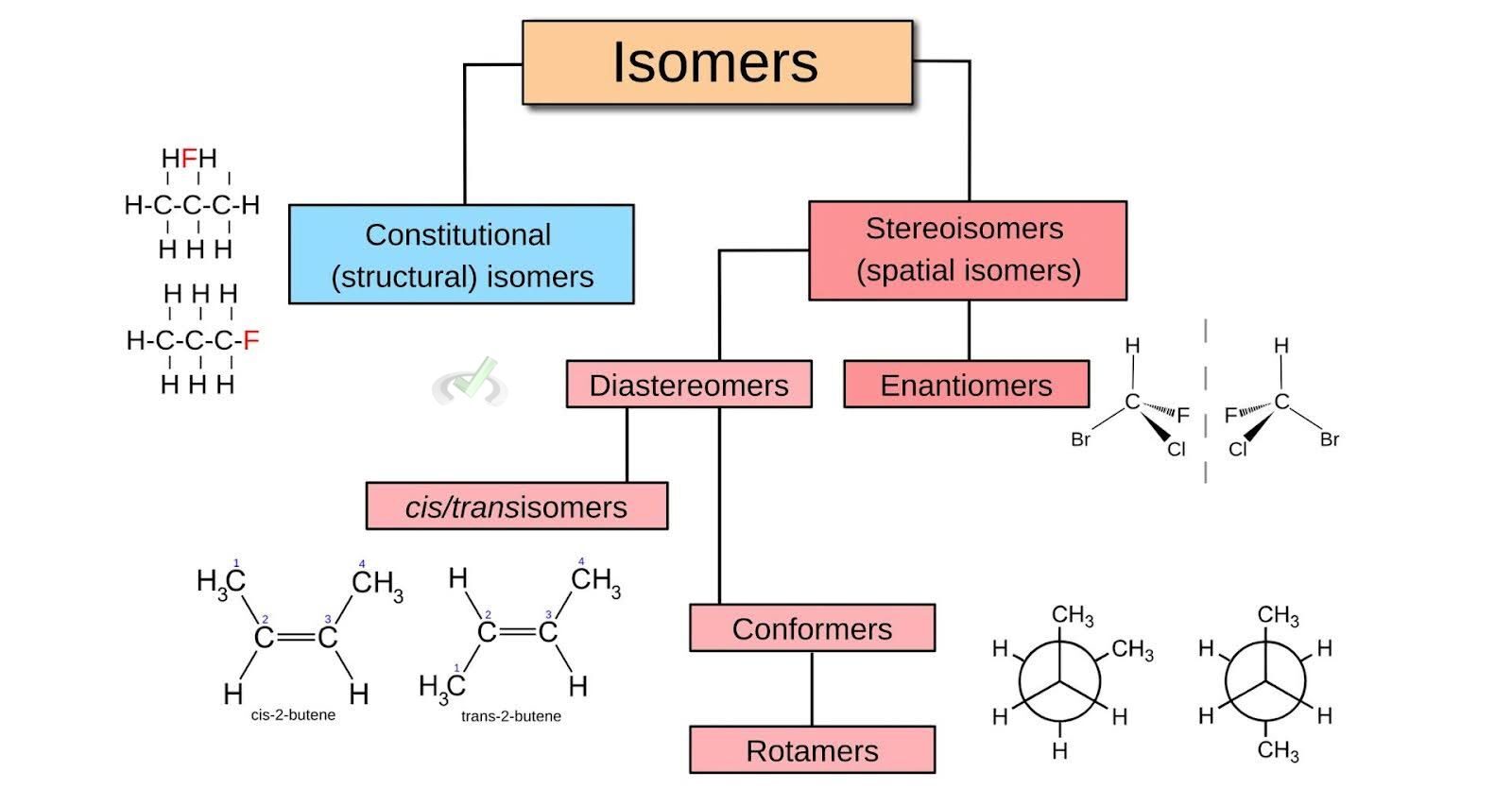
Isomers is broadly classified into two categories: structural isomers and stereoisomers. Each category can be further divided into subtypes.
- Structural Isomers: These isomers differ in the connectivity of their atoms.
- Stereoisomers: These isomers have the same connectivity but they differ in the spatial arrangement of their atoms.
Let's discuss these types of isomers in detail.
II. Structural Isomers
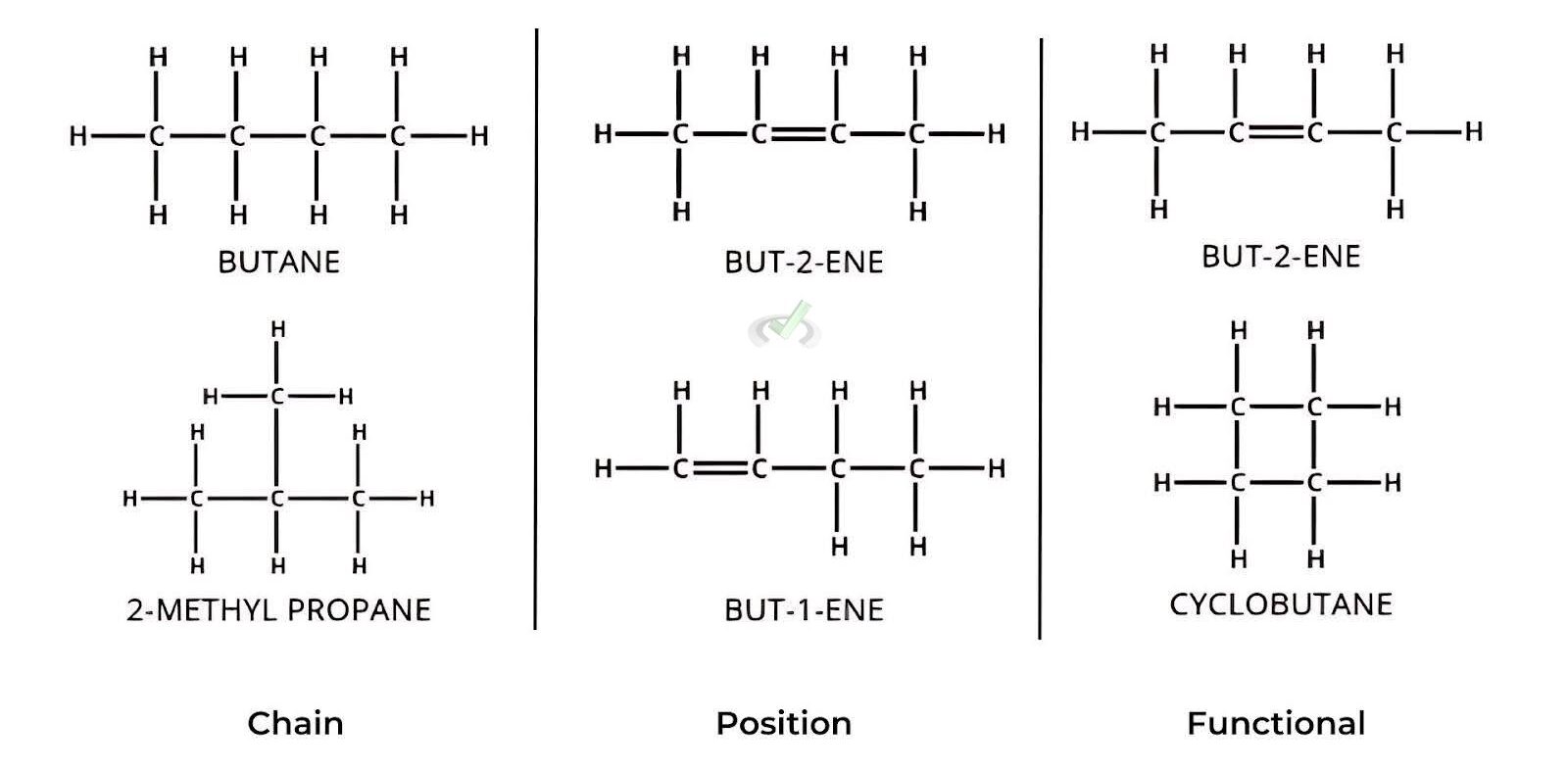
A. Chain Isomers
Chain isomers differ in the arrangement of the carbon skeleton. For example, butane (C4H10) can exist as:
- n-butane: A straight-chain structure
- Structural Formula: CH3-CH2-CH2-CH3
- Molecular Formula: C4H10
- isobutane: A branched-chain structure
- Structural Formula: (CH3)3CH
- Empirical Formula: C4H10
B. Position Isomers
Position isomers have the same carbon skeleton but differ in the position of a functional group or a substituent. For example, the position of a chlorine atom in chlorobutane can vary:
- 1-chlorobutane:
- Structural Formula: CH3-CH2-CH2-CH2-Cl
- Molecular Formula: C4H9Cl
- 2-chlorobutane
- Structural Formula: CH3-CH2-CH(Cl)-CH3
- Molecular Formula: C4H9Cl
C. Functional Group Isomers
Functional group isomers contain different functional groups. For instance, C3H6O can represent:
- Propanal (an aldehyde)
- Structural Formula: CH3-CH2-CH2-CH3
- Molecular Formula: C4H10
- Acetone (a ketone)
- Structural Formula: CH3-CO-CH3
- Molecular: C3H6O
III. Stereoisomers
A. Geometric Isomers
Geometric isomers occur due to the restricted rotation around a double bond or ring structure. They can be:
- cis-isomers: Substituents are on the same side.
- trans-isomers: Substituents are on opposite sides.
For example, 2-butene (C4H8) can be:
- cis-2-butene: Both methyl groups are on the same side of the double bond.
- Structural Formula: CH3-CH=CH-CH3
- Molecular Formula: C4H8
- trans-2-butene: Methyl groups on opposite sides of the double bond.
- Structural Formula: CH3-CH=CH-CH3
- Molecular Formula: C4H8
B. Optical Isomers
Optical isomers, or enantiomers, are non-superimposable mirror images of each other. They have a chiral center, usually a carbon atom bonded to four different groups.
Example: Lactic acid (C3H6O3) has two enantiomers:
- L-(+)-lactic acid
- Structural Formula: CH3-CH(OH)-COOH
- Empirical Formula: C3H6O3
- D-(-)-lactic acid
- Structural Formula: CH3-CH(OH)-COOH
- Empirical Formula: C3H6O3
IV. Identifying Isomers
A. Molecular Formula
The molecular formula gives the number of each type of atom in a molecule. However, it does not provide information about the structure. For example, C4H10 represents both butane and isobutane.
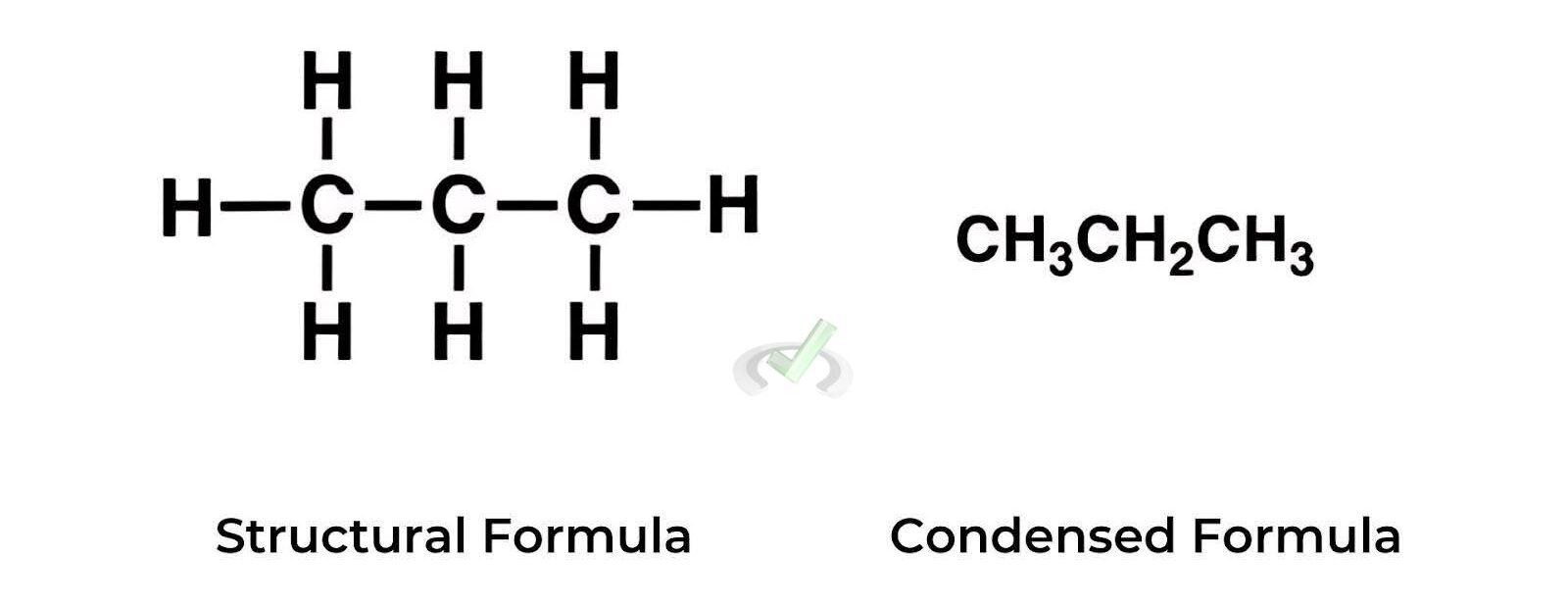
B. Structural Formulas
Structural formulas show the arrangement of atoms. They can be:
- Condensed structural formula: Groups atoms together.
- Displayed structural formula: Shows all atoms and bonds.
C. Stereochemical Representations
Stereochemical representations indicate the spatial arrangement of atoms. Examples include:
- Dash-wedge notation: Used for showing the 3D arrangement of atoms around a chiral center.
- Fischer projections: Used primarily for carbohydrates.
V. Bridging to Broader Concepts
A. Importance in Pharmaceuticals
Many drugs are chiral, and often, only one enantiomer is biologically active. For example, the drug thalidomide has one effective enantiomer, while the other causes severe congenital disabilities.
B. Biological Systems
Enzymes and receptors are chiral. Thus, the chirality of molecules affects how they interact with biological systems. Understanding isomers is crucial for drug design and biochemical research.
C. Industrial Applications
Geometric isomers can have different physical properties, like boiling points and solubilities. This affects their separation and purification in industrial processes.
VI. Wrap-Up and Key Terms
Understanding isomers is vital in organic chemistry. They include structural isomers and stereoisomers, each with unique properties and implications.
Key Terms
- Isomers: Compounds having the same molecular formula yet different in structures.
- Structural Isomers: Differ in the connectivity of their atoms.
- Stereoisomers: Same connectivity but different spatial arrangements.
- Geometric Isomers: Differ in the position of substituents around a double bond or ring.
- Optical Isomers (Enantiomers): Non-superimposable mirror images.
- Chiral Center: A carbon atom bonded to four different groups.
VII. Practice Questions
Sample Practice Question 1
What are isomers?
A) Molecules with the same molecular formula but different structures.
B) Molecules with different molecular formulas.
C) Atoms with different numbers of protons.
D) None of the above.
Ans. A
Isomers are compounds that have the same molecular formula.
Sample Practice Question 2
Which of the following is a structural isomer of butane?
A) Ethene
B) Propane
C) Isobutane
D) Methane
Ans. C
Isobutane (C4H10) is a structural isomer of butane. It has the same molecular formula but a different arrangement of carbon atoms.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
