Understanding the differences between structural (constitutional) isomers and stereoisomers is crucial in organic chemistry. These concepts help in grasping the complexity of molecules and their interactions. Let's explore these types of isomers, their characteristics, and examples to clarify their distinctions.
I. Basics of Isomers
A. What Are Isomers?
Isomers are molecules with similar molecular formula but different arrangements of atoms. This means they have the same number of each type of atom, but those atoms are connected or arranged differently.
For example, C₄H₁₀ can represent both butane and isobutane. They have different structures but the same molecular formula.
II. Structural (Constitutional) Isomers
A. Definition
Structural or constitutional isomers have similar molecular formula but they have different connectivity in their atoms. This means the atoms are bonded in different orders.
B. Types of Structural Isomers
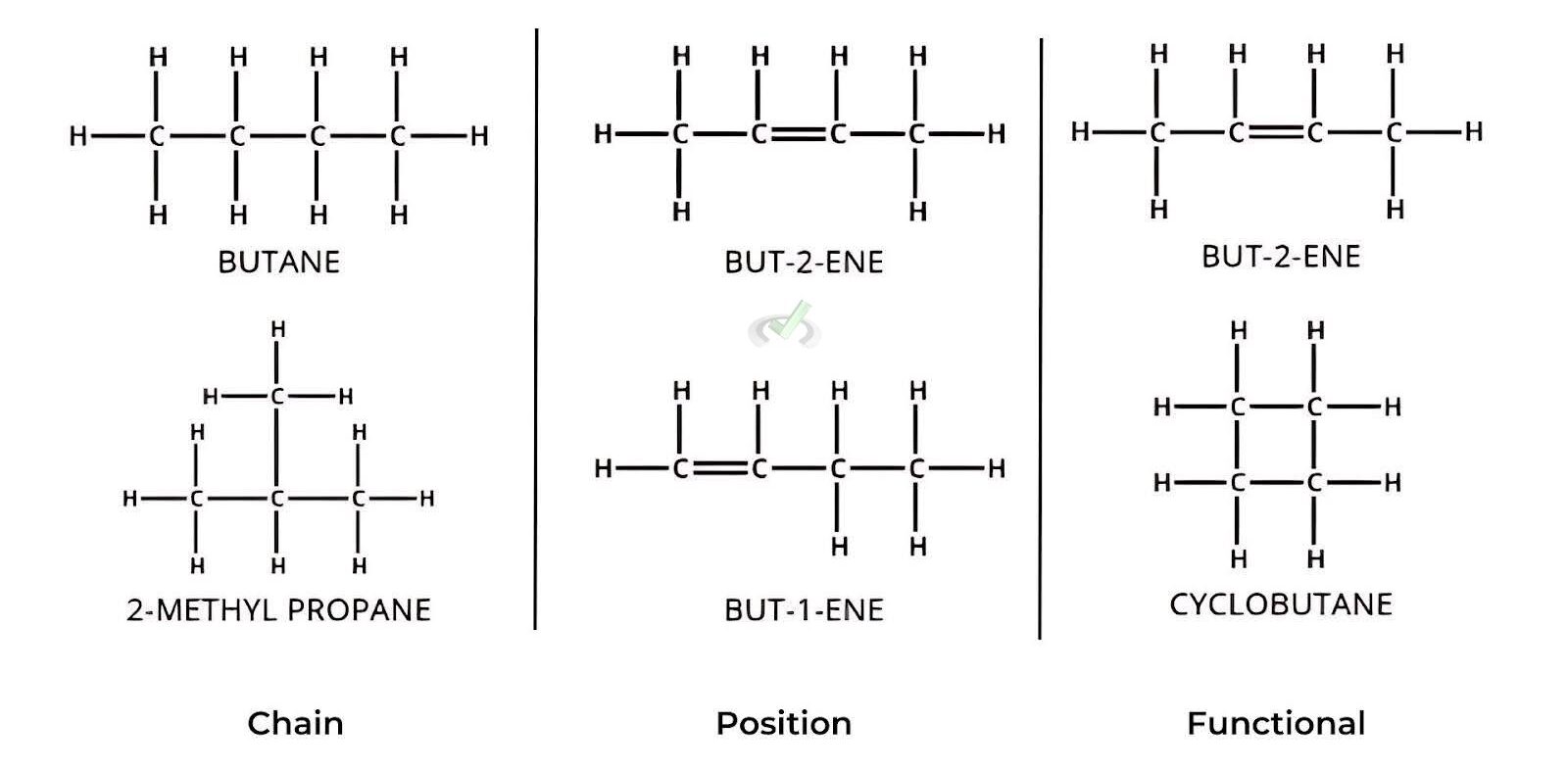
Chain Isomers
These isomers differ in the arrangement of the carbon chain. For example, butane (C₄H₁₀) can exist as n-butane, a straight chain, or 2-methylpropane, a branched chain.
Position Isomers
These isomers have the same carbon skeleton but differ in the position of a functional group. For instance, butene (C₄H₈) can be but-1-ene, with the double bond between the first and second carbon atoms, or but-2-ene, with the double bond between its second and third carbon atoms.
Functional Group Isomers
These isomers contain different functional groups. An example is but-2-ene (C₄H₈), which has a double bond, and cyclobutane (C₄H₈), which forms a ring structure.
C. Examples
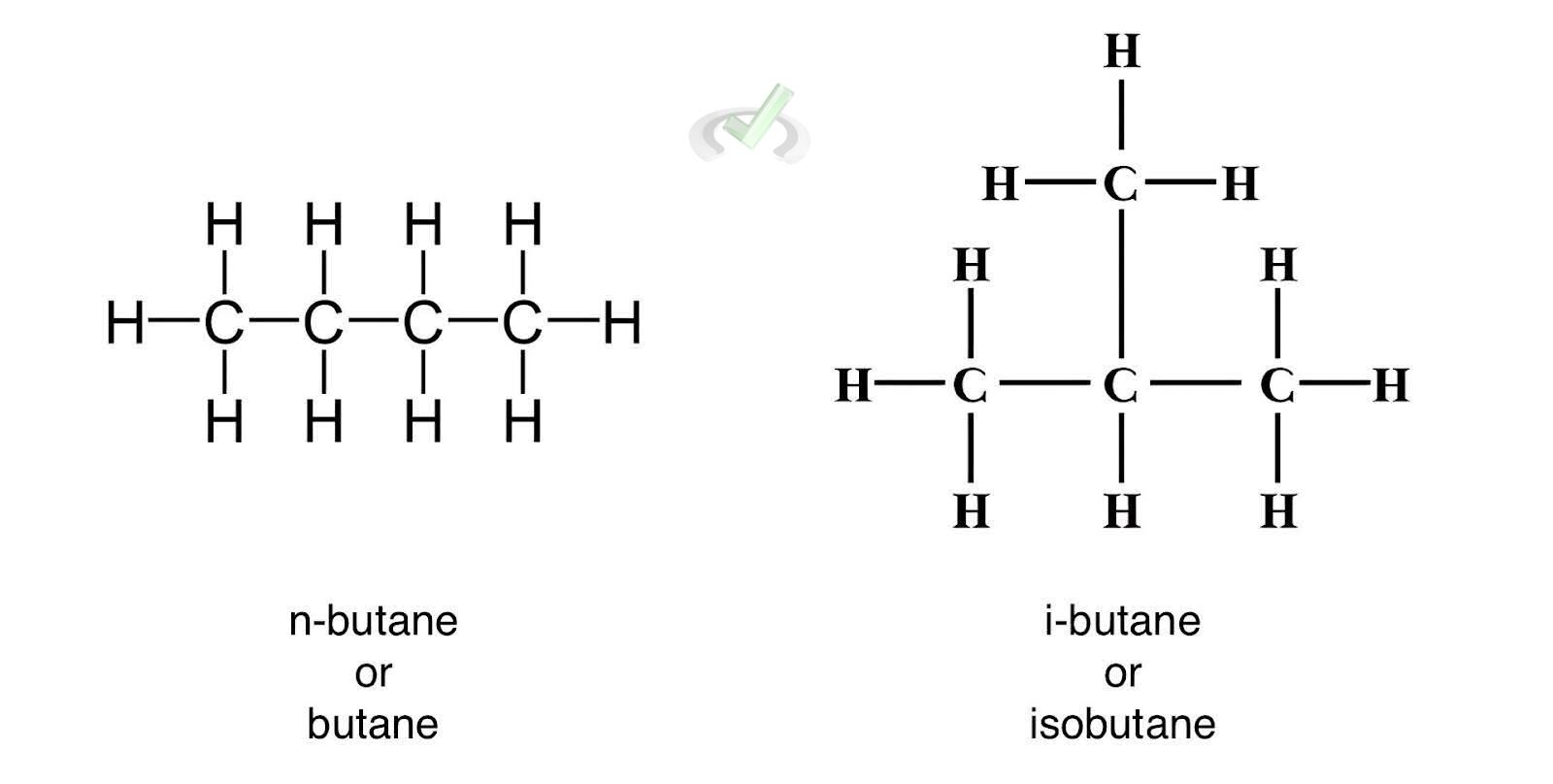
Butane and Isobutane:
Both have the molecular formula C₄H₁₀ but different structures.
- Butane: CH₃-CH₂-CH₂-CH₃
- Isobutane: (CH₃)₂CH-CH₃
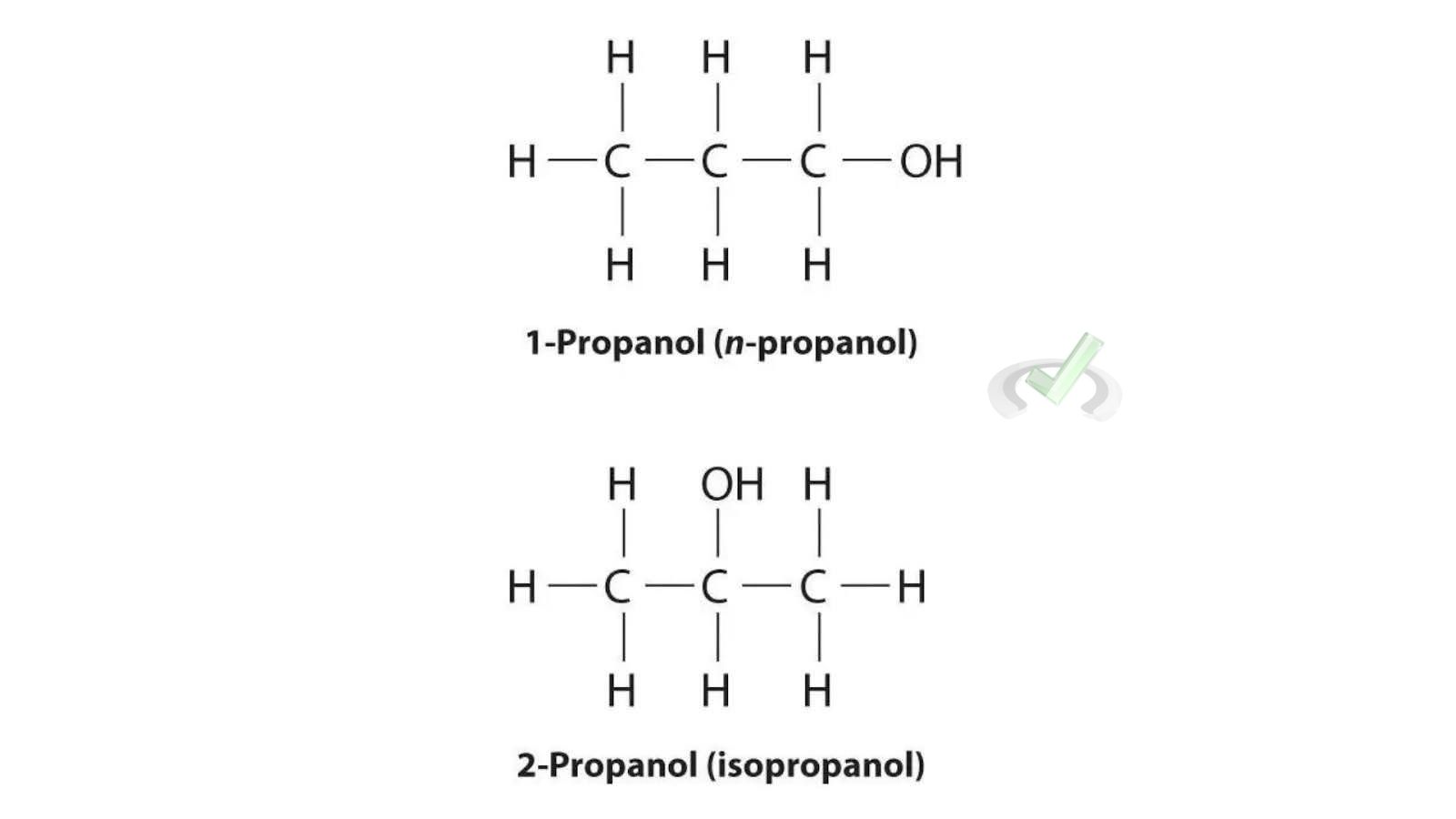
Propanol Isomers:
- 1-Propanol: CH₃-CH₂-CH₂-OH
- 2-Propanol: CH₃-CHOH-CH₃
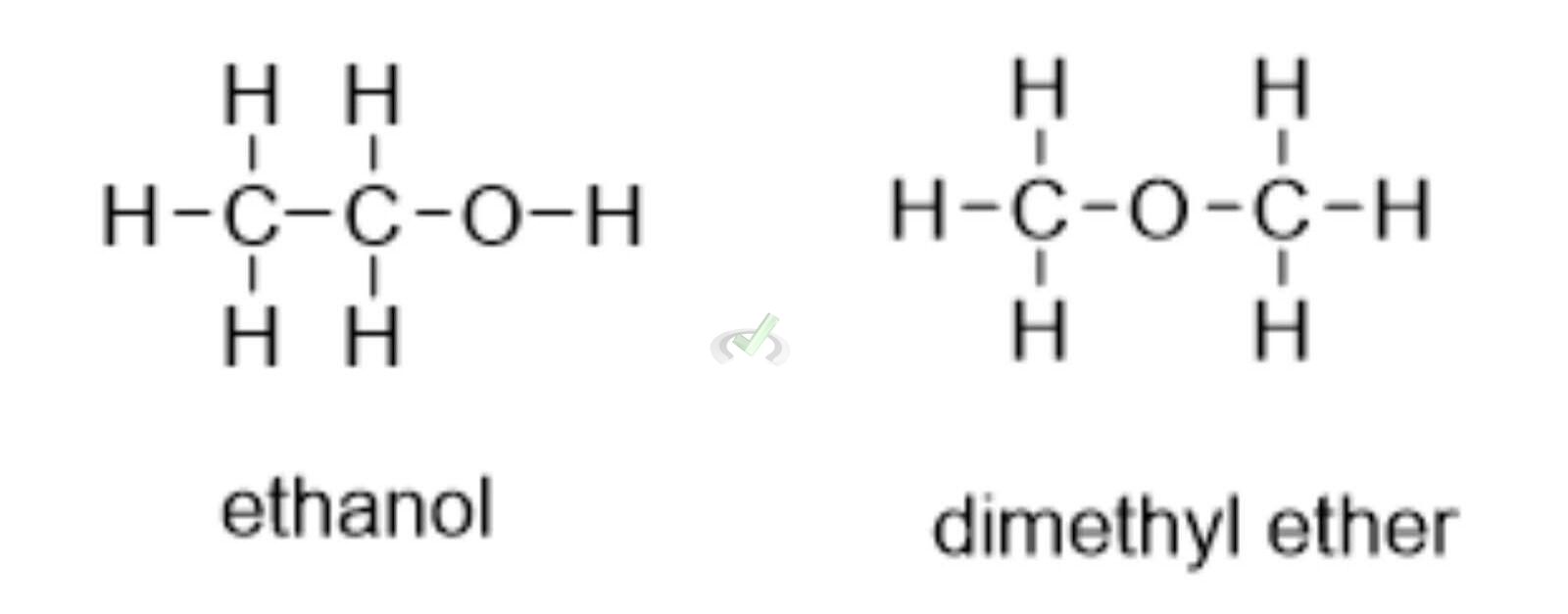
Ethanol and Dimethyl Ether:
- Ethanol: CH₃-CH₂-OH
- Dimethyl Ether: CH₃-O-CH₃
III. Stereoisomers
A. Definition
Stereoisomers have the same molecular formula and the same order of atom connectivity, but they differ in the spatial arrangement of atoms.
B. Types of Stereoisomers
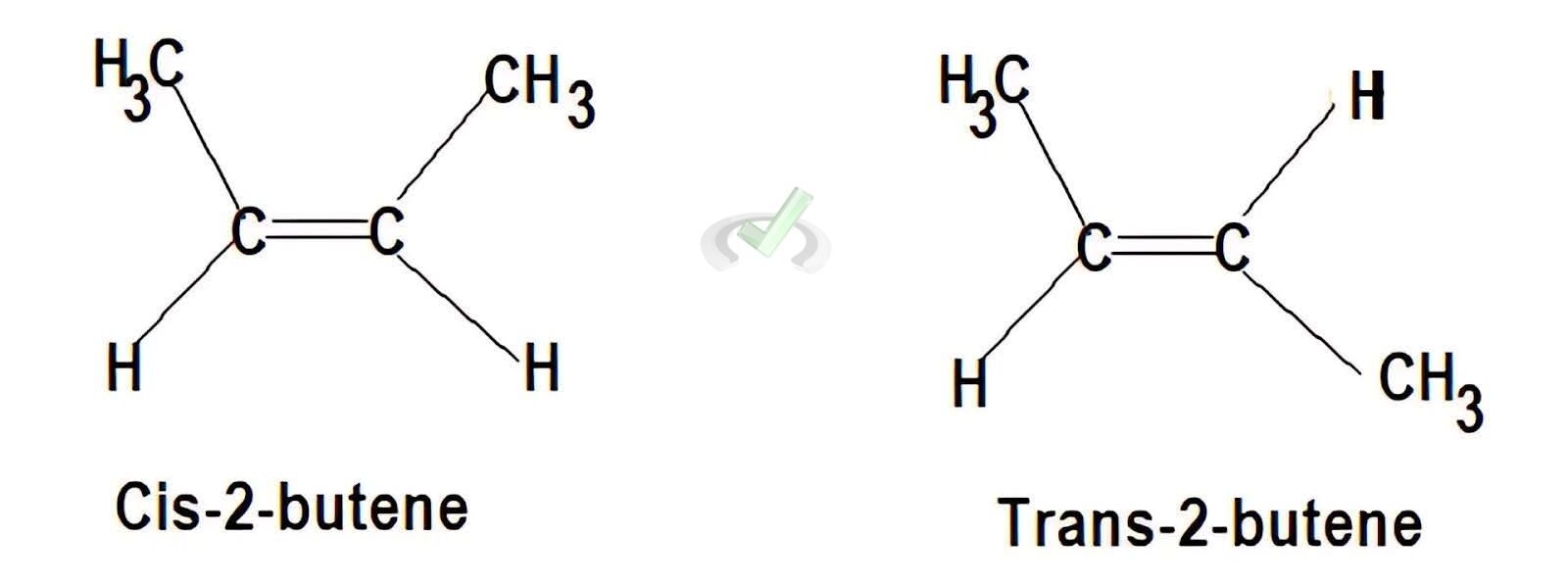
- Geometric Isomers (Cis-Trans Isomers): These isomers differ in the spatial arrangement of groups around a double bond or ring structure.
- Cis Isomer: Groups are on the same side.
- Trans Isomer: Groups are on opposite sides.
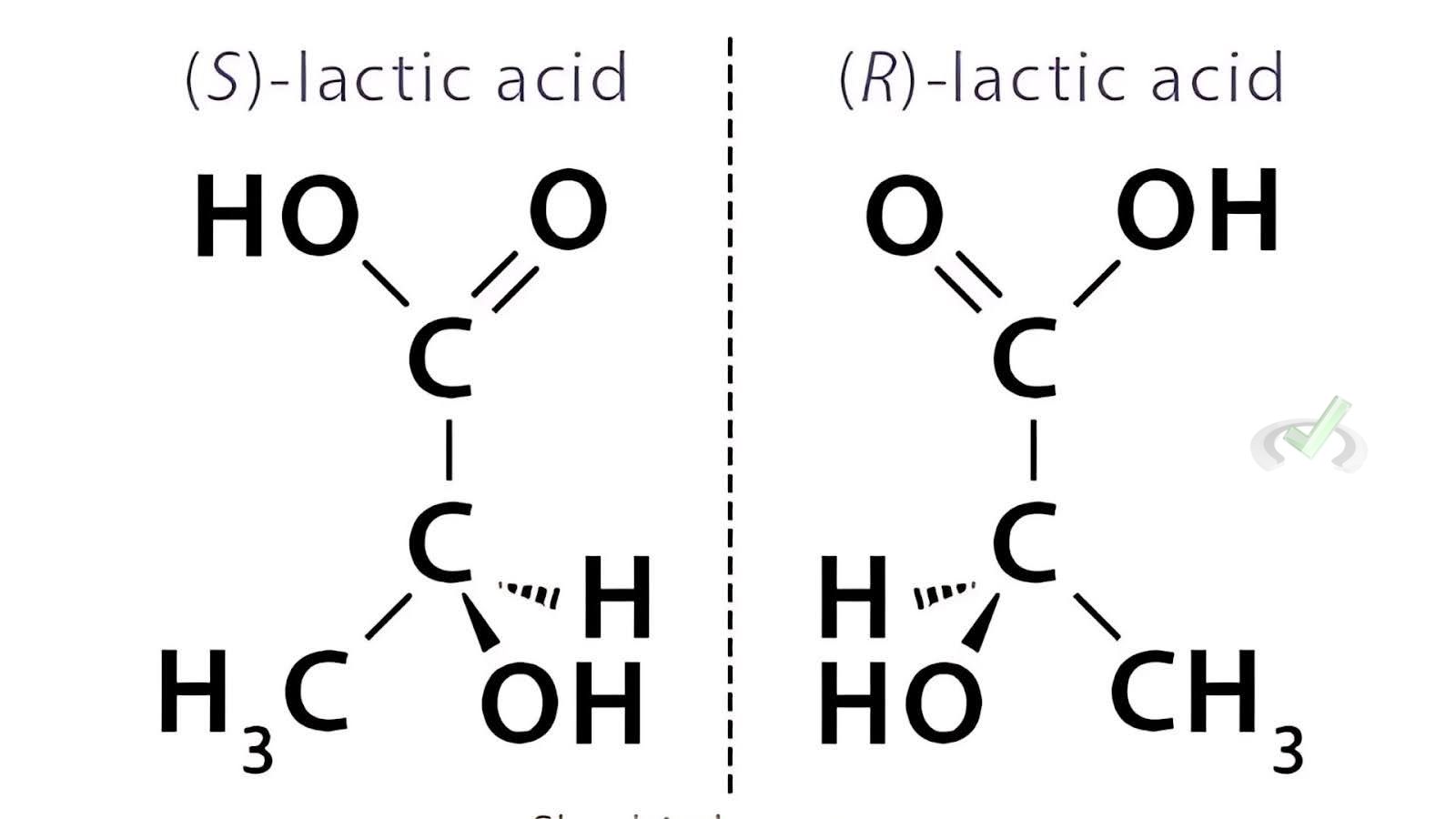
- Optical Isomers (Enantiomers): These are molecules that are non-superimposable mirror images of each other. They usually have at least one chiral center (a carbon atom with four groups attached).
C. Examples
Cis-Trans Isomers:
- 2-Butene (C₄H₈) can exist as:
- Cis-2-Butene: CH₃-CH=CH-CH₃ (groups on the same side)
- Trans-2-Butene: CH₃-CH=CH-CH₃ (groups on opposite sides)
- 2-Butene (C₄H₈) can exist as:
Optical Isomers:
- Lactic acid (C₃H₆O₃) has two enantiomers:
- (R)-Lactic Acid
- (S)-Lactic Acid
- Lactic acid (C₃H₆O₃) has two enantiomers:
IV. Differences and Characteristics
A. Structural Isomers
They have different physical and chemical properties. May have different boiling points, melting points, and reactivities.
Example: Due to their structural differences, butane and isobutane have different boiling points. Butane boils at -0.5°C, while isobutane boils at -11.7°C.B. Stereoisomers
They have the same physical properties but different spatial arrangements. It can have different chemical properties, especially in biological systems.
Example: (R)- and (S)-Lactic acid have different effects in biological systems due to their different shapes.V. Bridging Topics: Beyond Isomerism
A. Importance in Pharmaceuticals
Structural isomers can lead to drugs with different therapeutic effects. For example, ibuprofen is a common painkiller, and both isomers have the same effect. However, other drugs may have one isomer being active and the other being inactive or even harmful.
Stereoisomers are crucial in drug design. One enantiomer can be therapeutic, while the other may be harmful.
For instance, one enantiomer of the drug thalidomide caused birth defects. Meanwhile, the other was effective for treating morning sickness.
B. Enzymatic Reactions
Structural isomers can lead to drugs with different therapeutic effects. For example, ibuprofen is a common painkiller, and both isomers have the same effect. However, other drugs may have one isomer being active and the other being inactive or even harmful.
Stereoisomers are crucial in drug design. One enantiomer can be therapeutic, while the other may be harmful.
For instance, one enantiomer of the drug thalidomide caused birth defects. Meanwhile, the other was effective for treating morning sickness.
C. Advanced Organic Synthesis
Isomerism plays a significant role in complex organic synthesis. Chemists need to control which isomer they produce to ensure the desired effect of the molecule.
Control over stereochemistry is critical in synthesizing complex natural products and pharmaceuticals. This ensures that the correct isomer is used in treatments and medications.
VI. Differences and Characteristics VI. Wrap-Up and Key Terms
Understanding structural and stereoisomers is essential for mastering organic chemistry. Structural isomers differ in atom connectivity, while stereoisomers differ in spatial arrangement. These differences can have significant implications for both chemical properties and biological activities.
Key Terms
- Isomer: Molecules with the same molecular formula but different structures or arrangements.
- Structural (Constitutional) Isomer: Isomers with different atom connectivities.
- Stereoisomer: Isomers with the same connectivity but different spatial arrangements.
- Cis-Trans Isomerism: A type of stereoisomerism where groups are on the same or opposite sides of a double bond or ring.
- Optical Isomer (Enantiomer): Non-superimposable mirror images of molecules with chiral centers.
- Chiral Center: A carbon atom with four different groups attached, leading to optical isomerism.
VII. Practice Questions
Sample Practice Question 1
What is a structural (constitutional) isomer?
A) Molecules with different molecular formulas.
B) Molecules with the same molecular formula but different connectivity of atoms.
C) Molecules with the same molecular formula and connectivity but different spatial arrangements.
D) Molecules that are mirror images of each other.
Ans. B
Structural (constitutional) isomers have the same molecular formula but different atom connectivity. This means that the atoms are bonded in a different sequence. It results in different structures and properties.
Sample Practice Question 2
Which type of isomerism involves molecules that are mirror images of each other?
A) Chain isomerism
B) Geometric isomerism
C) Optical isomerism
D) Position isomerism
Ans. C
Optical isomerism involves molecules that are non-superimposable mirror images of each other. These are called enantiomers, which have chiral centers leading to different interactions with plane-polarized light and biological systems.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
