When NBA scouts and general managers are scouting for promising NBA prospects, they often have a variety of approaches and methods to decide which ones might be a good fit for a specific team
Just like choosing a specific player to fill a specific role for a team, organic chemists can use different laboratory techniques in order to separate and selectively isolate specific substances of interest!
After going through this chapter overview, you’ll have the basics of the different techniques that can be used to isolate specific substances.
Laboratory Techniques and Separations on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
This is one of the more emphasized organic chemistry topics on the MCAT – try and expect around 7-10 questions that’ll test this topic come test day!
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Laboratory Techniques and Separation
A great way to study and memorize these different laboratory techniques is to make a table or little figure describing each technique, its main features, what characteristics of the substances it tries to exploit, etc.
1. Rationale for Separation Laboratory Techniques
Just like when NBA scouts are looking for the right prospect among a group of players, organic chemists often also want to isolate a specific substance or molecule present within a mixture of substances and compounds!
The key point to really emphasize here is that for the most part, different compounds have different characteristics. As such, we can utilize the differing properties of compounds to our advantage and exploit these differences to separate substances and isolate specific molecules of interest.
To give a common daily example, when we strain cooked pasta noodles from the boiling water, we exploit their difference in matter: the water pours through the strainer leaving your pasta noodles ready to be covered in sauce!
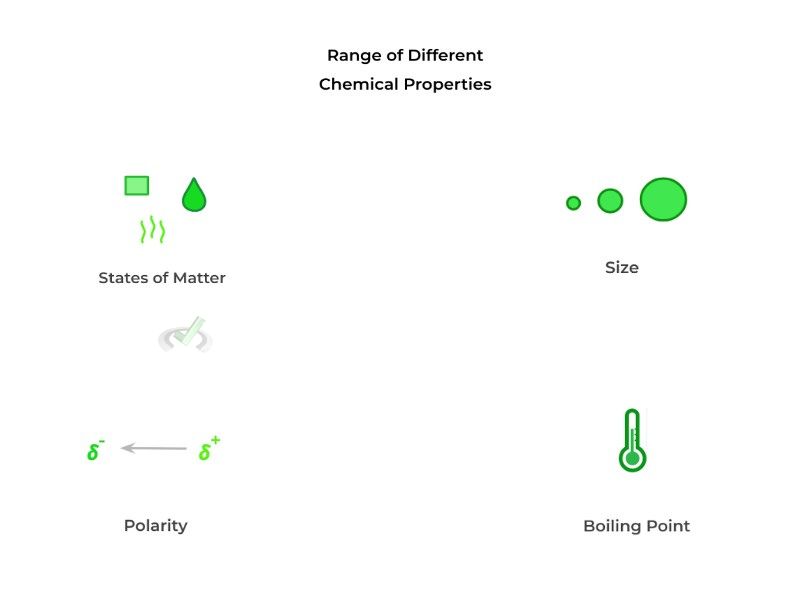
Above are some of the common characteristics of molecules that can be exploited in order to separate a mixture of substances and isolate a specific molecule of interest! You’ll see in the coming sections that different techniques will target different characteristics as their main strategy.
Full Study Notes : Rationale for Separation Laboratory Techniques
For more in-depth content review on rationale and logic behind the various separation methods used in the laboratory, check out these detailed lesson notes created by top MCAT scorers.
2. Extraction and Filtration
In the process of extraction, we can utilize the difference in polarity and solubility between molecules in order to separate them! Let’s give an example: suppose we have a mixture of sodium chloride (NaCl) and cholesterol and wish to separate them.
We can use their difference in polarity in order to separate them as NaCl is highly polar while cholesterol is a highly hydrophobic, nonpolar molecule. To do this, we need to put 2 immiscible solvents into a separatory funnel with them differing in density and polarity – this allows the solvents to be immiscible, meaning they won’t mix.
For this technique, we can use the phrase “like dissolves like” as a general principle to separate the molecules: polar molecules will dissolve in the polar solvent (called the aqueous phase) while nonpolar molecules will dissolve in the nonpolar solvent (called the organic phase).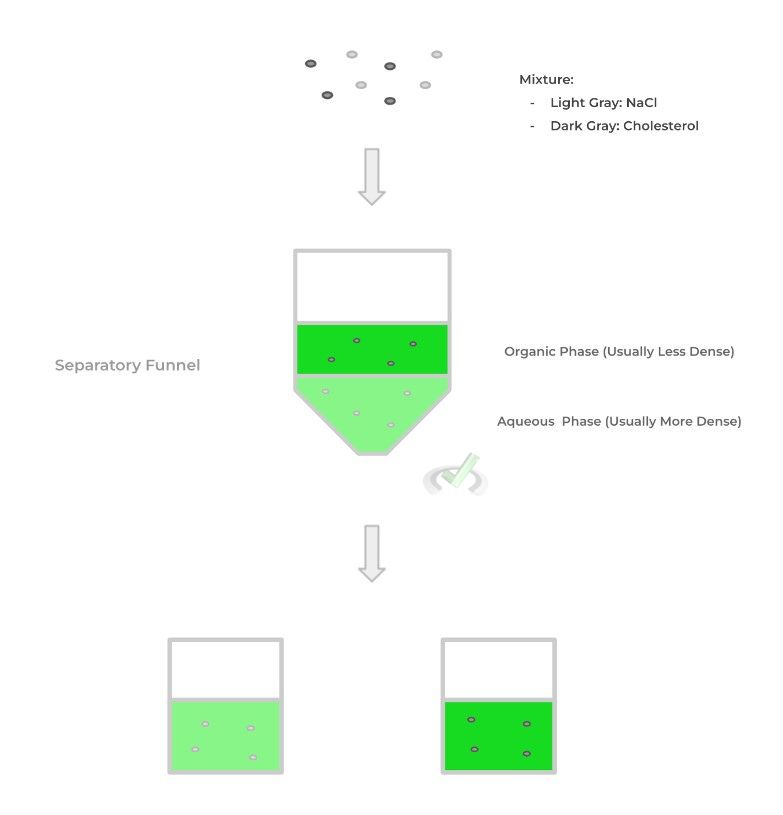
We insert the mixture into the separation flask and shake it in order for the substances to separate into the appropriate solvents. Because the solvents are different in density and are immiscible, you can pore out the denser solvent (usually the aqueous phase), which contains the polar molecule and separate the compounds!
Filtration takes advantage of the substance’s different states of matter in order to separate them and has a much easier process! We simply put a filtration paper over a funnel and pour the solid and liquid mixture.
The liquid portion of the mixture passes through while the solid residue is retained within the filtration paper. There are also different types of filtrations depending on which portion we’re trying to collect.
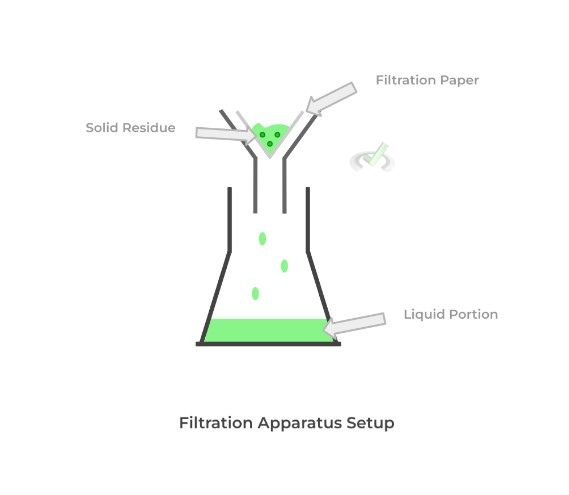
Gravity filtration simply uses the force of gravity to filter the mixture and is usually done to collect the liquid position. Vacuum filtration uses a vacuum to aid in filtration and is usually used to isolate the solid residue.
Full Study Notes : Extraction and Filtration
For more in-depth content review on extraction and filtration, check out these detailed lesson notes created by top MCAT scorers.
3. Types of Distillation
If we have a mixture of 2 liquids, we can use distillation techniques to separate them by taking advantage of their difference in boiling points! Recall that boiling point refers to the temperature when a liquid has enough average kinetic energy to transition from a liquid to a gaseous, vapor state.
In the process of distillation, we slowly heat the mixture of liquids in a flask. As such, the liquid with the lower boiling point should begin to boil and become a vapor first. The flask is connected to a condenser via a sidearm and is where the vapor will travel.
The condenser is surrounded by cold water, which allows for the condensation of the vapor back into a liquid, which is collected in another beaker. After replacing the beaker with a new one, we can repeat the process to obtain the other liquid.
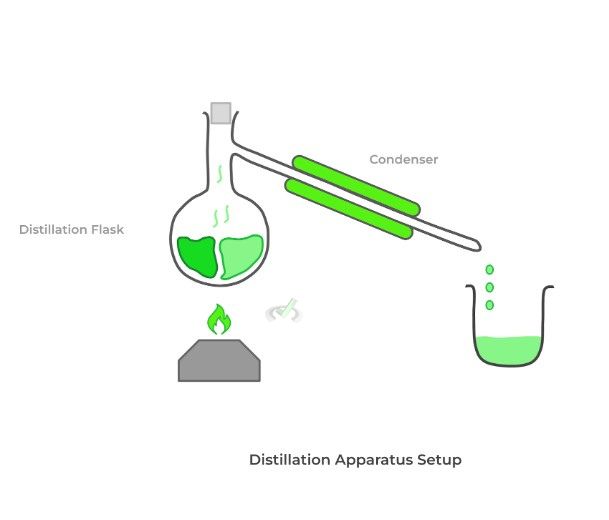
The distillation setup depicted above is good for simple distillation. On top of this, organic chemists also use fractional and vacuum distillation. We’ll cover their differences in detail in our article, but for now, know the following rules of thumb.
- Simple Distillation: Use when the liquids’ boiling points are at least 25 C˚ apart and less than 150 C˚
- Fractional Distillation: Use when the liquids’ boiling points are less than 25 C˚ apart
- Vacuum Distillation: Use when the liquids’ boiling points are higher than 150 C˚
Full Study Notes : Types of Distillation
For more in-depth content review on types of distillation, check out these detailed lesson notes created by top MCAT scorers.
4. Types of Chromatographies
Because there are many different types of chromatographies, there are many different characteristics we can exploit! Though there are many types of chromatographies, they all have 2 common components: a stationary and a mobile phase.
The stationary phase is the component that does not move and will bind to the molecules of interest. The mobile phase allows for the flow of the molecules in the mixture through the stationary phase, which attracts the molecules of interest allowing separation. Take a look at the step up from thin layer chromatography below!
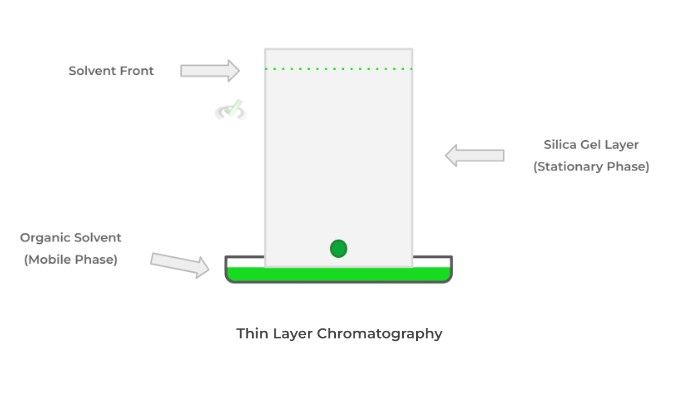
In thin layer chromatography (TLC), the simplest type of chromatography, we usually use a silica gel layer on a glass plate as our stationary phase – note that the silica gel is highly polar. Likewise, we usually use an organic solvent with weak polarity.
When we place the mixture sample on the sheet – called dotting – and submerge one end of the sheet in the organic solvent, the mobile phase begins to travel up the plate and can “carry” the mixture of substances.
Because the solvent is weakly polar, nonpolar molecules will travel further up on the sheet. Conversely, the polar molecules within the mixture will be attracted to the stationary phase and won’t move further up as much. As we can see, the phrase “like dissolves like” applies here as well.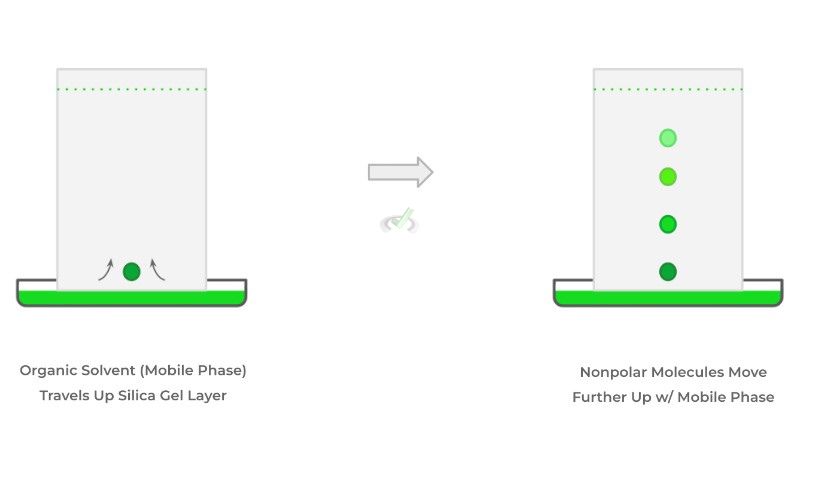
From this, other types of chromatography can be derived from the basics established from TLC. Another type of high yield chromatography technique you might be tested on is ion exchange chromatography which separates a mixture of charged ions!
In this case, the stationary phase will contain charged particles which will bind ions of the opposing charge. There are 2 types of ion exchange chromatographies: cation and anion exchange chromatography.
Cation exchange chromatography will bind positively charged cations which means the stationary phase will have negatively charged molecules. Conversely, anion exchange chromatography will bind negatively charged anions meaning the stationary phase will have positively charged molecules.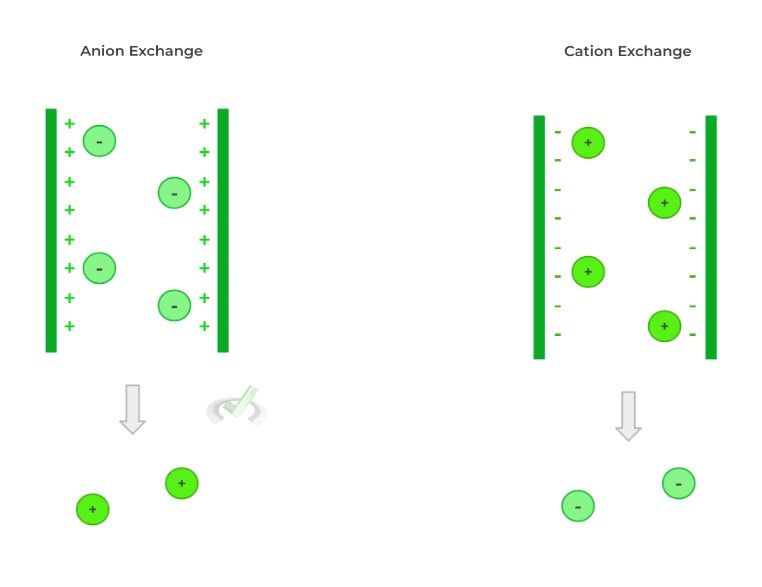
Another type of chromatography that is used is size exclusion chromatography. As indicated by its name, we’re utilizing the difference in the sizes of molecules to separate them!
In size exclusion chromatography, the column utilized contains beads which also has a column pore, as shown below. This slight detail is crucial to understand the pattern of how fast the substances will fall.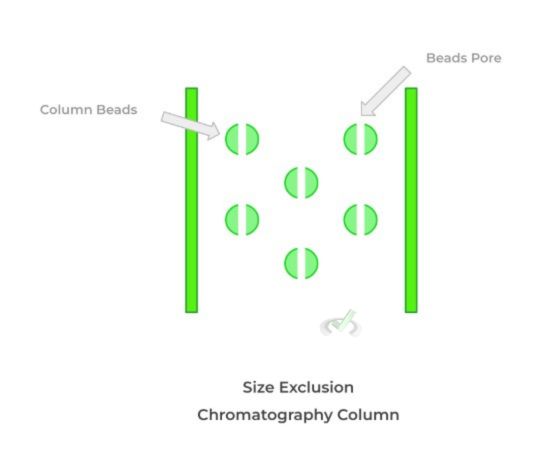
Although it might be counterintuitive at first, we see that actually, the larger molecules will actually elute first from the column while the smaller ones will be retained longer. This is because the larger molecules will not be trapped in the column pores within the beads and will instead travel “around the beads,” allowing them to travel faster.
The smaller molecules, by contrast, will get stuck within the column pores and be retained longer, which causes them to travel slower through the column.
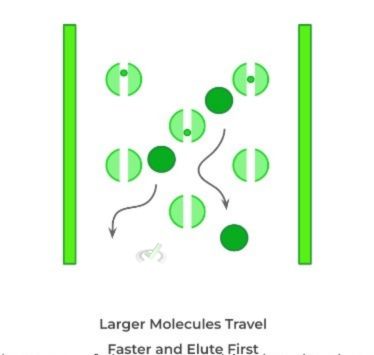
There are a few other types of chromatographies, but the above 3 are usually the most tested ones on the MCAT.
Full Study Notes : Types of Chromatographies
For more in-depth content review on types of chromatographies, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about laboratory techniques and separations in organic chemistry!
Term | Definition |
|---|---|
Extraction | Separation technique which allows for the separation of molecule via differences in their solubilities |
Aqueous Phase | Solvent used in extraction which is highly polar which allows for the dissolving of polar substances |
Organic Phase | Solvent used in extraction which is weakly polar which allows for the dissolving of nonpolar substances |
Filtration | Separation technique which separate substances via differences in their states of matter; Can be divided into gravity and vacuum filtration |
Simple Distillation | Separation technique which separates a mixture of liquids based on differences in their boiling point; Should only be done when boiling points are at least 25 C˚ apart and less than 150 C˚ |
Fractional Distillation | Type of distillation which should only be used when the difference in boiling points between the liquids is less than 25 C˚ |
Vacuum Distillation | Type of distillation which should only be used when the boiling points of the liquids is greater than 150 C˚ |
TLC Chromatography | A type of chromatography which allows for the separation of molecules based on their polarities |
Mobile Phase | The portion of chromatography which allows for the flow of the mixture through the stationary phase |
Stationary Phase |
The portion of chromatography which binds/attracts specific molecules of interest |
Ion Exchange Chromatography |
Type of chromatography which allows for the separation of anions and cations |
Size Exclusion Chromatography |
Type of chromatography which allows for the separation of substances based on their size |
Additional FAQs - Laboratory Techniques and Separation in Organic Chemistry on the MCAT
What are Lab Techniques on the MCAT?
What is Distillation? – MCAT
What is Chromatography? – MCAT
What is Thin Layer Chromatography? – MCAT
When the organic solvent moves up the layer, the more polar molecules are attracted and retained in the silica gel stationary phase while nonpolar molecules will continue to travel further up with the organic solvent.
What is Gas Liquid Chromatography? – MCAT
The mixture of volatile compounds can then be separated based on many factors, such as polarity, molecular weight, etc., depending on the characteristics of the stationary phase.
Additional Reading Links – Study Notes for Laboratory Techniques and Separations on the MCAT
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Alcohols and Ethers on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
