In scientific research, separating different compounds from a mixture is important. One of the most effective techniques for this is chromatography.
This method uses the different ways compounds interact with materials to separate and analyze mixtures. Let’s explore chromatography in detail, as it is fundamental in chemistry, biology, and medicine.
I. Introduction to Chromatography
Chromatography is a laboratory technique used to separate mixtures into their individual components. It relies on the different interactions between the compounds in the mixture and the materials used in the chromatography process.
II. Key Concepts in Chromatography
Understanding the basic principles of chromatography is essential before diving into the specific types. Each type relies on similar concepts but applies them in different ways.
Mobile Phase and Stationary Phase
- Mobile Phase: This is the solvent that moves through the chromatography system. It carries the mixture's components with it.
- Stationary Phase: This material stays fixed inside the chromatography system. The mixture's components interact with this phase differently, causing them to separate.
For example, in paper chromatography, the mobile phase is the solvent moving up the paper, and the stationary phase is the paper itself.
Retention Factor (Rf)
The retention factor is a number that represents how far a compound travels in a particular chromatography system. It helps identify compounds by comparing their Rf values to known standards.
For example, if a compound travels 2 cm while the solvent travels 10 cm, the Rf value is 0.2.
Adsorption and Partition
- Adsorption: This occurs when compounds stick to the surface of the stationary phase. For example, in column chromatography, compounds may stick to the silica gel in the column.
- Partition: This happens when compounds dissolve in the stationary phase. For instance, in paper chromatography, compounds can dissolve in the water present in the paper fibers.
III. Types of Chromatography
Each type of chromatography has specific applications and uses, making it suitable for different scenarios. Let's explore the main types.
Paper Chromatography
Paper chromatography is one of the simplest forms. It utilizes a strip of paper as the stationary phase.
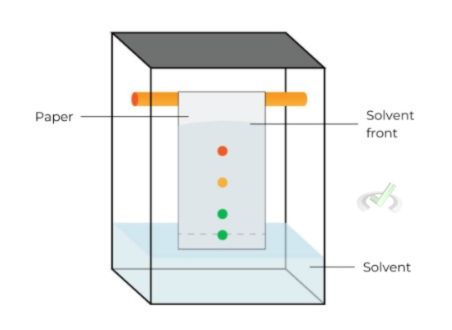
- Procedure: A spot of the mixture is placed on the paper. The paper is then placed in a solvent. The solvent moves up the paper, carrying different compounds at different rates.
- Applications: It is used in teaching labs and for simple separations, like identifying plant pigments.
Thin-Layer Chromatography (TLC)
TLC is similar to paper chromatography but uses a thin layer of solid material, such as silica gel, on a glass or plastic plate.
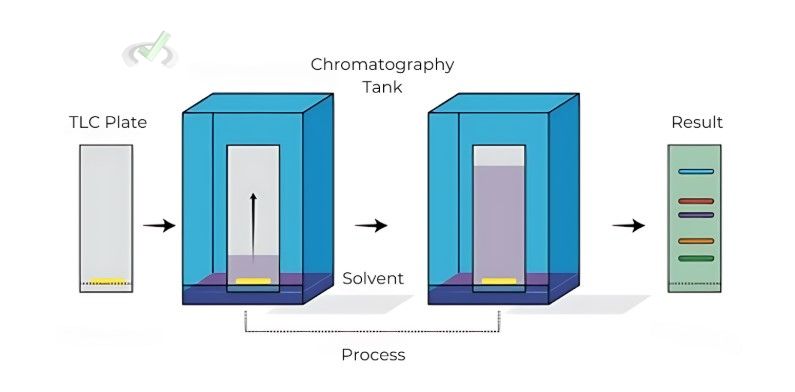
- Procedure: A spot of the mixture is placed on the TLC plate. The plate is then placed in a solvent. As the solvent goes up the plate, it separates the mixture's components.
- Applications: TLC is used for monitoring reactions, checking the purity of compounds, and identifying substances.
Column Chromatography
Column chromatography uses a column filled with a solid adsorbent as the stationary phase.
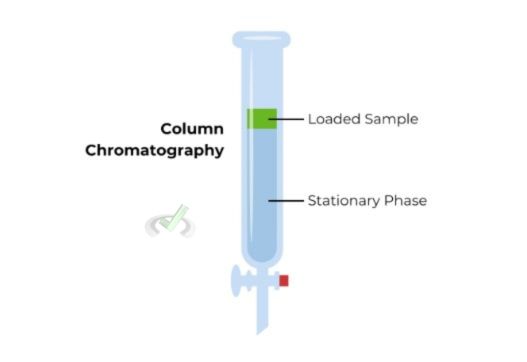
- Procedure: First, the mixture is poured into the column, and then a solvent is introduced, which proceeds to flow through the column. As the solvent moves, different compounds in the mixture travel at varied rates within the column.
- Applications: It is used to purify individual chemical compounds from mixtures. For example, it can separate different pigments from a plant extract.
Gas Chromatography (GC)
Gas chromatography separates compounds based on their boiling points and interactions with the stationary phase inside a column.
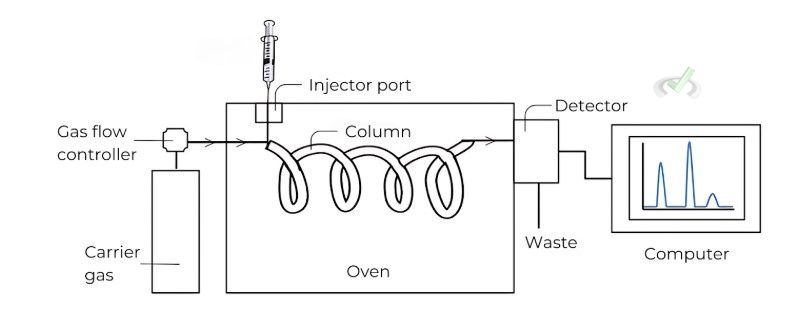
- Procedure: The mixture is vaporized and carried by an inert gas through a column. Compounds separate based on their interactions with the column material.
- Applications: GC is used in forensic science, environmental testing, and the pharmaceutical industry. For instance, it can analyze the components of a blood sample to detect drugs.
High-Performance Liquid Chromatography (HPLC)
HPLC is a highly efficient type of chromatography that uses high pressure to push solvents through a column.
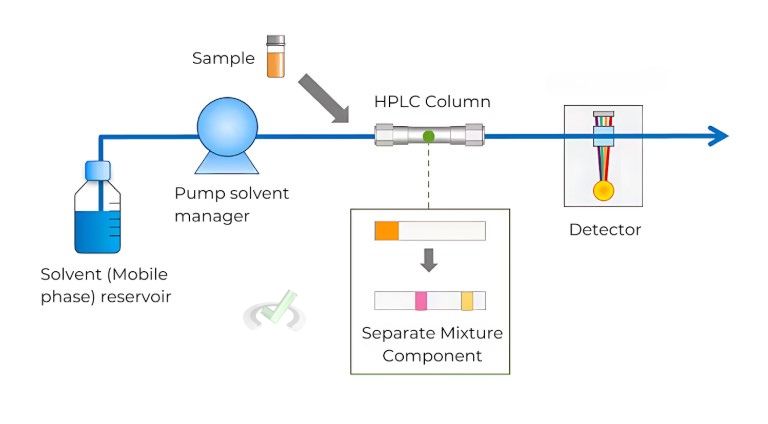
- Procedure: The mixture is dissolved in a solvent and pumped through a column filled with a solid adsorbent. The compounds separate as they move through the column.
- Applications: HPLC is used in drug testing, food analysis, and quality control. It can detect contaminants in food products or measure the purity of a pharmaceutical drug.
IV. Applications of Chromatography
Chromatography has numerous applications in various fields. These applications demonstrate the practical importance of understanding different chromatography techniques.
Pharmaceutical Industry
In the pharmaceutical industry, chromatography is used to analyze and purify drugs. It ensures the quality and safety of medications. For example, HPLC can measure a drug's concentration of active ingredients.
Environmental Testing
Chromatography helps detect pollutants in air, water, and soil. It is essential for monitoring environmental health and safety. GC can detect volatile organic compounds (VOCs) in air samples.
Forensic Science
Forensic scientists use chromatography to analyze substances found at crime scenes. It helps identify drugs, explosives, and other chemicals. TLC can identify drug residues on clothing or other surfaces.
Food Industry
In the food industry, chromatography tests the quality and safety of food products. It helps detect contaminants and ensures compliance with regulations. HPLC can identify pesticides in fruits and vegetables.
V. Scientific Implications of Chromatography
Understanding chromatography extends beyond its basic applications. It connects with broader scientific concepts and techniques, making it relevant to various fields of study.
Chemical Analysis
Chemical analysis techniques, including chromatography, are vital in organic chemistry. Understanding these techniques is essential for analyzing reaction products and identifying unknown compounds. For instance, analyzing the purity of synthesized compounds in organic chemistry labs.
Biochemistry
In biochemistry, chromatography separates and studies biomolecules like proteins and nucleic acids. This technique is crucial for understanding metabolism, genetics, and molecular biology.
Analytical Chemistry
Analytical chemistry techniques, including chromatography, are essential for conducting experiments and analyzing data. This understanding is critical for laboratory work in various scientific fields. For example, quantifying the concentration of glucose in blood samples.
VI. Wrap-Up and Key Terms
Understanding chromatography involves several key concepts and principles. Let's review:
Key Terms
- Mobile Phase: The solvent that moves through the chromatography system.
- Stationary Phase: The material that stays fixed inside the chromatography system.
- Retention Factor (Rf): A number representing how far a compound travels in a chromatography system.
- Adsorption: When compounds stick to the surface of the stationary phase.
- Partition: When compounds dissolve in the stationary phase.
VII. Practice Questions
Sample Practice Question 1
What is the mobile phase in chromatography?
A. The material that stays fixed inside the chromatography system
B. The solvent that moves through the chromatography system
C. The number representing how far a compound travels
D. The compound that dissolves in the stationary phase
Ans. B
The mobile phase is the solvent that moves through the chromatography system. It carries the mixture's components with it.
Sample Practice Question 2
Which type of chromatography uses a gas as the mobile phase?
A. Paper Chromatography
B. Thin-Layer Chromatography (TLC)
C. Column Chromatography
D. Gas Chromatography (GC)
Ans. D
Gas chromatography uses a gas as the mobile phase in order to separate compounds based on their boiling points, as well as interactions with the stationary phase.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
