Understanding nucleophiles and electrophiles is key to mastering organic chemistry. These concepts help explain how and why chemical reactions occur.
Nucleophiles are species that donate an electron pair. On the other hand, electrophiles are species that accept an electron pair. Here, we'll explore the basics and delve into examples and applications.
I. Basics of Nucleophiles and Electrophiles
A. Nucleophiles
Nucleophiles are chemical species that donate an electron pair to form a chemical bond in a reaction. They are often negatively charged ions or neutral molecules with lone pairs of electrons.
Examples:
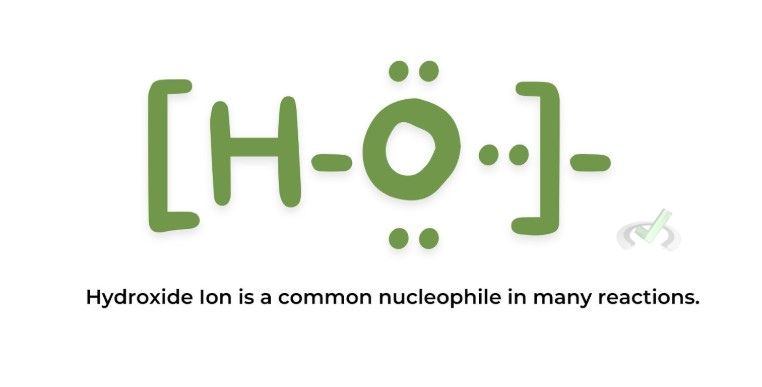
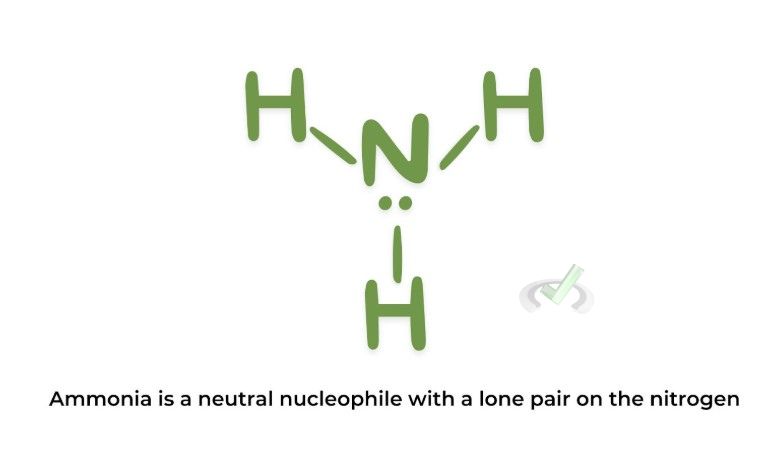

B. Electrophiles
Electrophiles are chemical species that accept an electron pair to form a chemical bond. They are often positively charged ions or neutral molecules with an electron-deficient atom.
Examples:
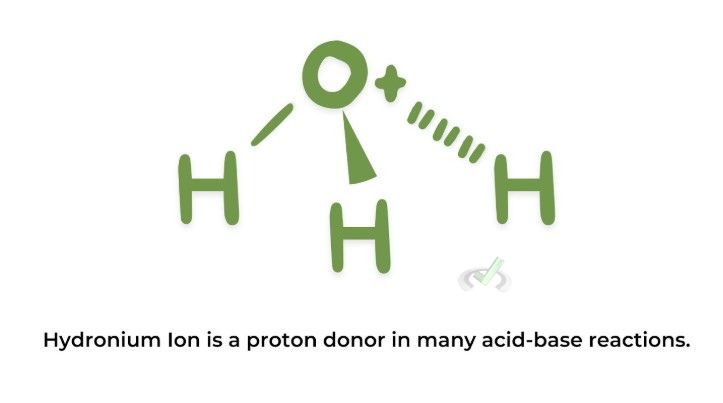
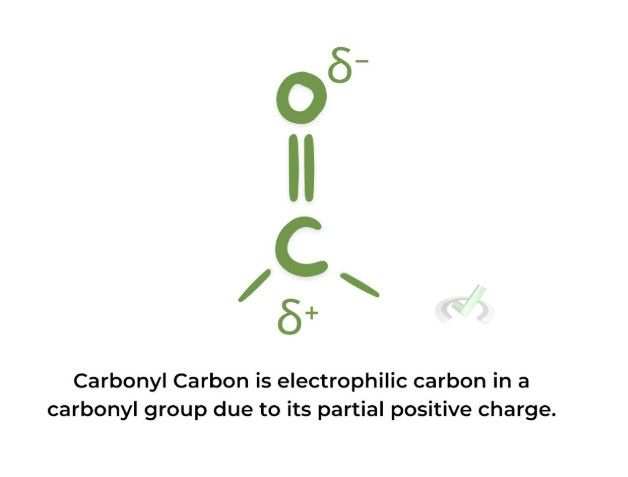

II. Characteristics and Reactions
A. Nucleophiles
Properties: Nucleophiles are attracted to positively charged or electron-deficient areas.
Reactivity: Nucleophilicity is influenced by charge, electronegativity, and solvent.
- Charge: Negatively charged nucleophiles are generally more reactive.
- Electronegativity: Less electronegative atoms make better nucleophiles. This is because they hold onto electrons less tightly.
- Solvent: Polar aprotic solvents (e.g., DMSO, acetone) increase nucleophilicity. Meanwhile, polar protic solvents (e.g., water, alcohols) can decrease it.
B. Electrophiles
Properties: Electrophiles are attracted to negatively charged or electron-rich areas.
Reactivity: Electrophilicity is influenced by charge, electron-withdrawing groups, and structure.
- Charge: Positively charged electrophiles are more reactive.
- Electron-Withdrawing Groups: Groups that pull electron density away from the electrophilic center increase reactivity.
- Structure: The geometry and accessibility of the electrophilic site affect reactivity.
III. Examples of Nucleophilic and Electrophilic Reactions
A. Nucleophilic Substitution Reactions
SN1: A two-step reaction where the leaving group departs before the nucleophile attacks. Common in tertiary alkyl halides. The first step forms a carbocation, which is a positively charged ion. The nucleophile then attacks the carbocation.
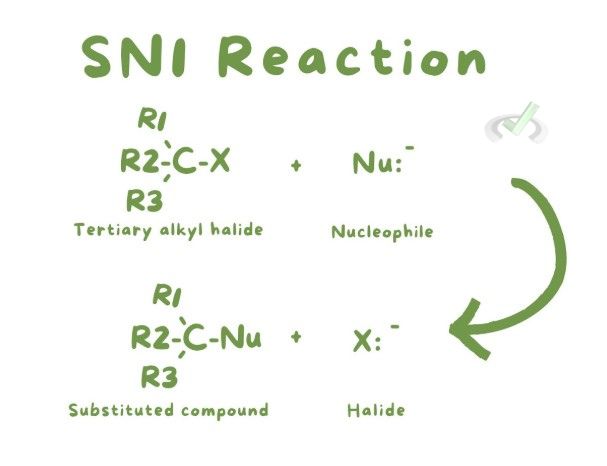
SN2: A one-step reaction where the nucleophile attacks as the leaving group departs. Common in primary alkyl halides. The nucleophile attacks from the opposite side of the leaving group, leading to an inversion of configuration.
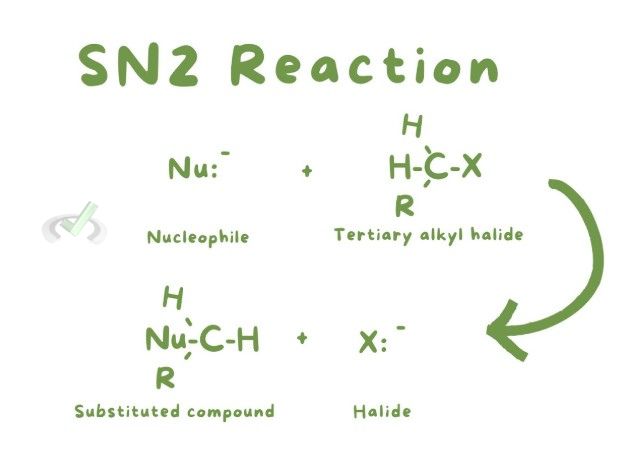
B. Addition Reactions
Nucleophilic Addition to Carbonyls:
Aldehydes and Ketones: Nucleophiles add to the carbonyl carbon, forming an alcohol.
Example: Cyanide ion (CN⁻) added to formaldehyde (H₂C=O) to form a cyanohydrin.
C. Electrophilic Addition Reactions
Electrophilic Addition to Alkenes:
Alkenes: Electrophiles add to the double bond of alkenes, forming new products.\
Example: Addition of bromine (Br₂) to ethene (C₂H₄) forming 1,2-dibromoethane.
IV. Connecting Concepts in Organic Chemistry
A. Synthesis and Mechanisms
Understanding nucleophiles and electrophiles aids in designing synthesis pathways. For example, knowing that a carbonyl carbon is electrophilic helps plan nucleophilic addition reactions.
B. Functional Group Transformations
Transformations often involve nucleophiles and electrophiles. For instance, converting an alcohol to a ketone might involve oxidation (removing electrons) followed by nucleophilic addition.
C. Biochemical Relevance
In biochemistry, many reactions involve nucleophiles and electrophiles. Enzymes often facilitate these reactions. For example, amino acids in proteins can act as nucleophiles. It attacks electrophilic carbon atoms in substrates.
V. Linking Organic Chemistry Fundamentals to Comprehensive MCAT Topics
A. Reaction Mechanisms and Kinetics
Understanding nucleophiles and electrophiles helps in predicting reaction mechanisms and rates. For instance, the stability of intermediates like carbocations (in SN1) or transition states (in SN2) determines the reaction pathway and speed.
B. Acid-Base Chemistry
Nucleophiles and electrophiles relate to Lewis acid-base theory. Nucleophiles are Lewis bases because they donate an electron pair. Electrophiles are Lewis acids because they accept an electron pair. This concept links to broader acid-base chemistry covered in the MCAT.
C. Biological Molecules
In biological systems, nucleophiles and electrophiles are involved in synthesizing and functioning DNA, RNA, and proteins. For example, the nucleophilic attack by the hydroxyl group of a ribose sugar on a phosphate group forms the backbone of DNA and RNA.
VI. Wrap-Up and Key Terms
Understanding nucleophiles and electrophiles is fundamental in organic chemistry. Here are some key concepts to remember:
- Nucleophiles: Electron pair donors, often negatively charged or neutral with lone pairs.
- Electrophiles: Electron pair acceptors, often positively charged or neutral but electron-deficient.
- Reactivity: Influenced by charge, electronegativity, solvent (for nucleophiles), and electron-withdrawing groups (for electrophiles).
VII. Practice Questions
Sample Practice Question 1
What type of species is a nucleophile?
A. Electron pair acceptor
B. Electron pair donor
C. Proton donor
D. Proton acceptor
Ans. B
A nucleophile is a species that donates an electron pair to form a new chemical bond during a reaction. This electron donation characteristic is why nucleophiles are called electron pair donors. Nucleophiles tend to be negatively charged or neutral with lone pairs of electrons.
Sample Practice Question 2
Which of the following is a good nucleophile?
A. H₂O
B. OH⁻
C. CH₄
D. NH₄⁺
Ans. B
Hydroxide ion (OH⁻) is a good nucleophile. This is because it has a negative charge, which makes it eager to donate its electrons. Water (H₂O) has lone pairs but is neutral, making it a weaker nucleophile than OH⁻. Methane (CH₄) lacks lone pairs of electrons to donate, and ammonium ion (NH₄⁺) is positively charged. This makes it an electrophile rather than a nucleophile.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
