Though oxidation and reduction reactions may not be the first things you think about when talking about organic chemistry, you’ll find that a lot of organic and general chemistry processes involve some aspect of redox reactions.
If you’ve covered (or have yet to cover) important metabolic pathways such as glycolysis, the citric acid cycle, etc., you’ll see that all these biochemical processes involve the transfer of electrons, which is why it’s so important to study the basics of oxidation-reduction reactions!
This chapter overview should give you a good introduction to redox reactions in the context of organic chemistry. Let’s get started!
Redox Reactions on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
When talking about redox reactions in organic chemistry, think of it more as an application topic. Rather than giving you how many questions the MCAT will likely ask, we view redox reactions in organic chemistry as topics where you’ll apply what you learn in order to approach passage content and apply these concepts when learning other topics!
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Redox Reactions
Luckily, redox reactions in the context of organic chemistry are fairly simple and even much simpler based on what the MCAT covers! You won’t be expected to calculate the oxidation state in organic chemistry passages (though in general chemistry, you will).
Rather, you have to focus on understanding whether an organic molecule is being oxidized or reduced based on what atoms it’s bonded to as we’ll touch on shortly.
1. General Overview of Redox Reactions
Let’s briefly review the basics of redox reactions. Recall that redox reactions are reactions that involve the transfer of electrons between 2 molecule species. The term “redox” is derived from the two electron transfer processes: oxidation and reduction.
Oxidation refers to when a species loses electrons, while reduction refers to when a species gains electrons. To remember this, use the popular mnemonic OILRIG which stands for “oxidation is losing (electrons), reduction is gaining (electrons)”.
2 additional terms that are important when discussing redox reactions are the oxidizing and reducing agent, which we assign to the molecules. It’s important to note, however, that their names are a bit counterintuitive to what actually occurs: the oxidizing agent is the species that is reduced while the reducing agent becomes oxidized.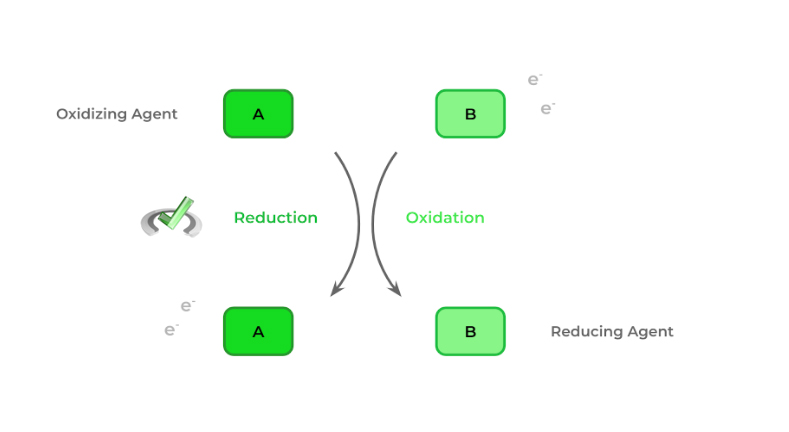
Let’s look at a real life example of a redox reaction! The conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate in glycolysis is an example of a redox reaction!
Here, glyceraldehyde-3-phosphate acts as the reducing agent and is oxidized to 1,3-bisphosphoglycerate. Conversely, the cofactor NAD+ acts as the oxidizing agent and is reduced to NADH.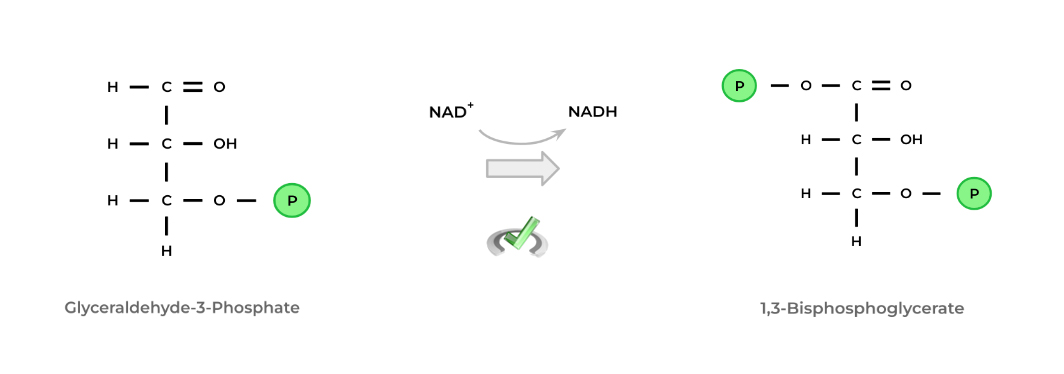
Notice that when NAD+ was reduced to NADH, it gained a hydrogen atom. Conversely, when glyceraldehyde-3-phosphate was oxidized to 1,3-bisphosphoglycerate, the carbonyl carbon became bonded to an oxygen atom on a phosphate group.
Full Study Notes : Basics on Redox Reactions
For more in-depth content review on we’ll examine why this change in bonding to specific atoms causes oxidation and reduction to occur, check out these detailed lesson notes created by top MCAT scorers.
2. Redox Reactions in the Context of Organic Chemistry
Studying redox reactions in the context of organic chemistry is more of an “application topic". In this context, you’ll instead take a more general approach and see how the bonding changes of a molecule will cause it either to be oxidized or reduced!
Because organic chemistry focuses on carbon based molecules, we usually look at how the changes in bonding to a carbon atom either cause oxidation or reduction. Bonding changes refer to the changes in the atoms that are bonded to the carbon.
A great general rule of thumb to know come test day is that molecules become oxidized when a carbon atom becomes bonded to a more electronegative atom (i.e. O, N, etc.).
Likewise, a molecule becomes reduced when a carbon atom becomes bonded to a less electronegative atom – in most cases for organic chemistry, this involves increasing bonds to hydrogen atoms. Let’s look at the figure below to see why!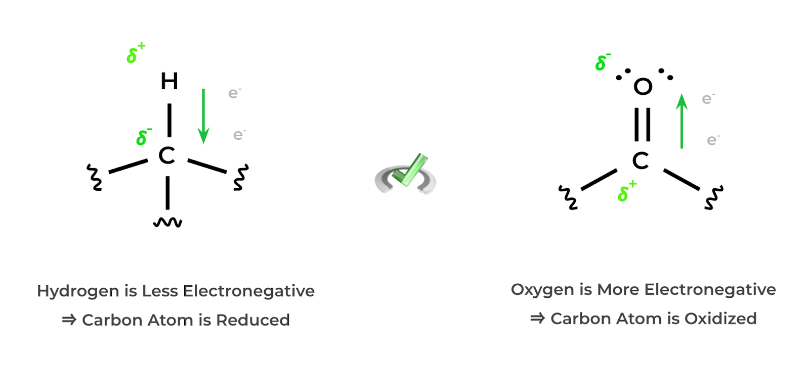
As shown, we can even think about this simply via the dipoles generated by the difference in electronegativity! Because we have a difference in electronegativity between the atoms, the electrons are unevenly distributed and shared!
If hydrogen is bonded, carbon is slightly more electronegative and attracts more electrons towards it, causing it to be“reduced”. If oxygen is bonded, its higher electronegativity attracts electrons away from the carbon, causing it to be “oxidized”.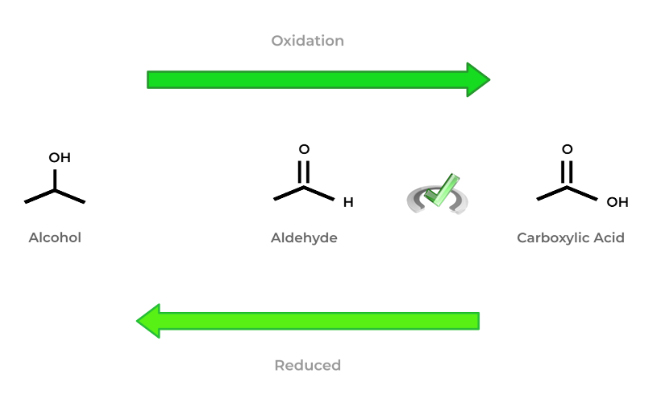
The above figure also shows that this rule also applies to the number of bonds! In this case, the more bonds carbon has to oxygen, the more oxidized it is!
Make sure to get comfortable with the above figure! While memorizing it is fine, try and rather focus on how the principles and general rules we discussed above result in the trend shown in the figure!
Full Study Notes : Redox Reactions in the Context
For more in-depth content review on redox reactions on organic chemistry, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about redox reactions in organic chemistry!
Term | Definition |
|---|---|
Redox Reactions | A type of chemical reaction characterized by the transfer of electrons between 2 species |
Oxidation | Refers to the loss of electrons in a molecule |
Reduction | Refers to the gain of electrons in a molecule |
Oxidizing Agent | The species in a redox reaction that gets reduced |
Reducing Agent | The species in redox reaction that get oxidized |
Additional FAQs - Redox Reactions in Organic Chemistry on the MCAT
Are Redox Reactions on the MCAT?
However, when placed in the context of organic chemistry, redox reactions take a more general approach. You’ll likely have to determine whether a molecule becomes oxidized or reduced based on the changes in bonding for that molecule.
How Do You Remember Oxidizing and Reducing Agents? – MCAT
The oxidizing agent is the species in the redox reaction that actually gets reduced, while the reducing agent is the species that gets oxidized.
What are the 5 Redox Reactions? – MCAT
These include combination/synthesis, decomposition, single substitution, double substitution, and combustion reactions!
How Do You Calculate the Oxidation Number? – MCAT
Additional Reading Links – Study Notes for Redox Reactions Organic Chemistry on the MCAT
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Alcohols and Ethers on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
