Our previous article on thermodynamics defined thermodynamics as the movement of heat. We study thermodynamics to understand how energy can be transferred without work exertion. It’s an important topic in physics as it explains how heat moves and why it behaves the way it does.
I. The Definition of Heat
We usually think of heat as synonymous with temperature. While the two terms are related, in thermodynamics, we consider them to have completely different meanings. Consider a molten ball dunked inside a huge container of cold water.
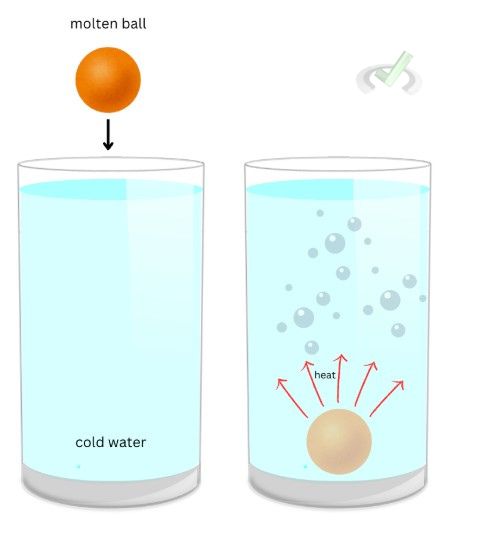
The molten ball has a higher temperature than that of the water. Water will react as the molten ball falls into the container, and steam or air bubbles will come out because the ball and water both have different temperatures. The heat coming from the ball will naturally flow towards its surroundings so it can maintain a balanced temperature. Heat is the energy that flows between the molten ball and the water. Temperature is how much energy is present in the object. As the heat flows out of the ball and into the water particles, the temperature rises because of the energy transfer to its surroundings.
Heat is an extensive property. This means it’s a property dependent on how much substance is present. If the ball is dropped in a small container of cold water, heat will spread faster than if put in a big container because of how little water is in the smaller container. Temperature is an intensive property because it does not rely on how much stuff is inside an object. Before dropping a molten ball, the two containers will always have the same temperature despite having different sizes.
We use temperature to measure how hot an object is. This “hotness” results from heat transferring from one body to another. In the MCAT, you will be tested by how much you know about the fundamentals of thermodynamics as it can explain the importance of homeostasis or balance that can be observed in many areas like biological processes, drug interactions, mechanisms, and other technologies used in medicine, such as MRI scans.
II. Why Heat Moves
We talked about thermal energy as the kinetic energy on a microscopic scale. All objects have tiny particles that make up the whole thing. These tiny particles move by vibration, and the result of this vibration is called thermal energy. When an object is heated, in this case, the water surrounding the ball increases thermal energy due to heat transfer from the ball to the water. This transfer will continue until it reaches equilibrium or the balance in the energy or temperature between the ball and the surrounding water.
As in everything in the world, things break loose when there is no balance. In the same logic, heat moves from one thing to another because of the lack of balance between the energy of two objects. In our example, heat transfers from the ball to the water because there is no balance between it and its surroundings. There is also the concept of entropy, which is the law that states that the disorder in a system always increases, and when two objects have different energy, the energy present in the more disordered object (molten ball) will always move towards the less disordered object (surrounding water) to increase the whole disorderness of the system.III. How Heat Moves
Now that we know why heat moves, let’s explore the ways in which it moves. Heat moves in three main ways: Conduction, Convection, and Radiation.
Conduction
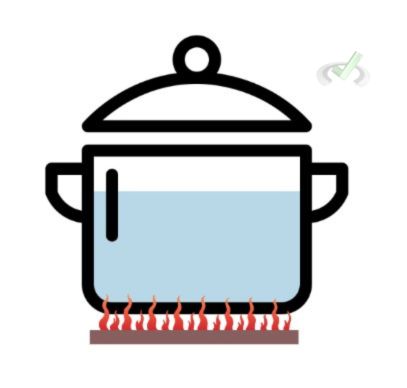
This mode of heat transfer requires direct contact from one object to another. The previous situation involving the molten ball is an example of conduction because the metal ball is in direct contact with the surrounding water. Heat directly moves from the ball to its surroundings due to this point of contact. Another good example is heating a pot of water. As the stove is turned on, the flame in contact with the pot transfers heat directly to the bottom of the pot. It can also be defined as the movement of heat without movement among objects.
When something is thermally conductive, it means that it can transfer heat quickly. A metal spoon, for example, is more thermally conductive than a wooden spoon because the former will heat up faster.Convection
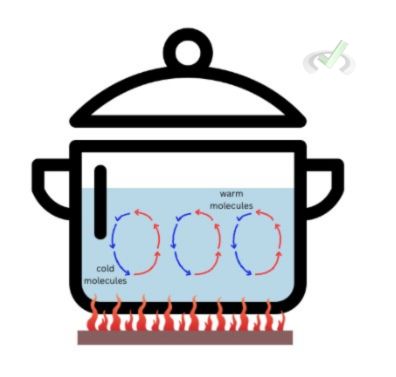
Convection is a heat transfer mode involving fluid movement (a liquid or gas). Due to the temperature gradient, this movement can be seen as a circulation fluid. As a pot of water heats up, the hotter part at the bottom will naturally move up as the cooler water on top moves down.
Convection may be natural or forced. Heating a pot of water is an example of forced convection, as it uses a medium to heat up. During the daytime, much warmer air near the land rises as much cooler air sinks, creating the same circulation of warm air rising and cool air sinking–this is an example of natural convection since the flow of heat is caused by natural forces.Radiation

While conduction and convection involve some form of contact for heat transfer, radiation is a mode of heat transfer that does not need a point of physical contact or a medium to induce heat. Radiation refers to the transfer of heat involving the release of energy as moving subatomic particles or the release of electromagnetic waves as a pot of water boils from heating; the heat from the pot of water transfers to the surrounding air. The change in temperature surrounding the pot indicates that heat is transferred due to the subatomic reaction occurring in the water molecules–in this case, the molecules reach enough heat to release some form of infrared radiation that the naked eye cannot see. The heat coming from the sun is also an example of radiation.
IV. Conclusion
The role of heat in thermodynamics is crucial in understanding the science behind energy transfer. Heat is the movement of energy, and it’s the energy that does not work. Unlike work, where we can see a large force through our eyes, the movement of heat happens on a smaller scale. Learning this is crucial as it explains why drinking a hot beverage makes us break a sweat even if we’re drinking it on a cold day or how MRI scans can see things inside our bodies without opening them up. Everything in nature seeks balance, but it also needs some form of bigger disorder. In our next lesson, we’ll look at what this disorder is, how certain laws apply to understanding the movement of heat, and the not-so-pretty equations that simplify these laws.
V. Key Terms
- Conduction - A mode of heat transfer that involves direct contact with the heat source
- Convection - A mode of heat transfer involving the movement of a fluid
- Entropy - State of disorder in a system
- Extensive Property - A property that is dependent on the amount of matter
- Heat - thermal energy that is transferred
- Intensive Property - A property that does not depend on the amount of matter
- Radiation - A type of heat transfer that moves through electromagnetic waves
- Temperature - Measure of kinetic energy in a system







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
