After the tragic events of the COVID-19 pandemic — which the WHO has now called off as a public health crisis — we’re all probably in need of strengthening and re-establishing some of the social bonds that were slightly weakened during that time.
Just like us, when deprived of social interaction for a long time, reactive elements also look to react with each other in order to form chemical bonds. Most of the time, they’ll do this in order to increase their stability, as we’ll talk about later.
This chapter overview will equip you with the basics for different types of chemical bonds and the important chemical interactions that take place between particles and molecules. Let’s go!
Bonding and Chemical Interactions on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try to expect around 2-4 questions that can appear on the MCAT covering stoichiometric topics, probably with a higher emphasis on calculation based questions!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Bonding and Chemical Interactions
Bonding and chemical interactions are some of the most high yield topics in regard to general chemistry. As such, it’s safe to say that there should be an added emphasis on this chapter in addition to other high yield chapters.
1. Types of Chemical Bonds
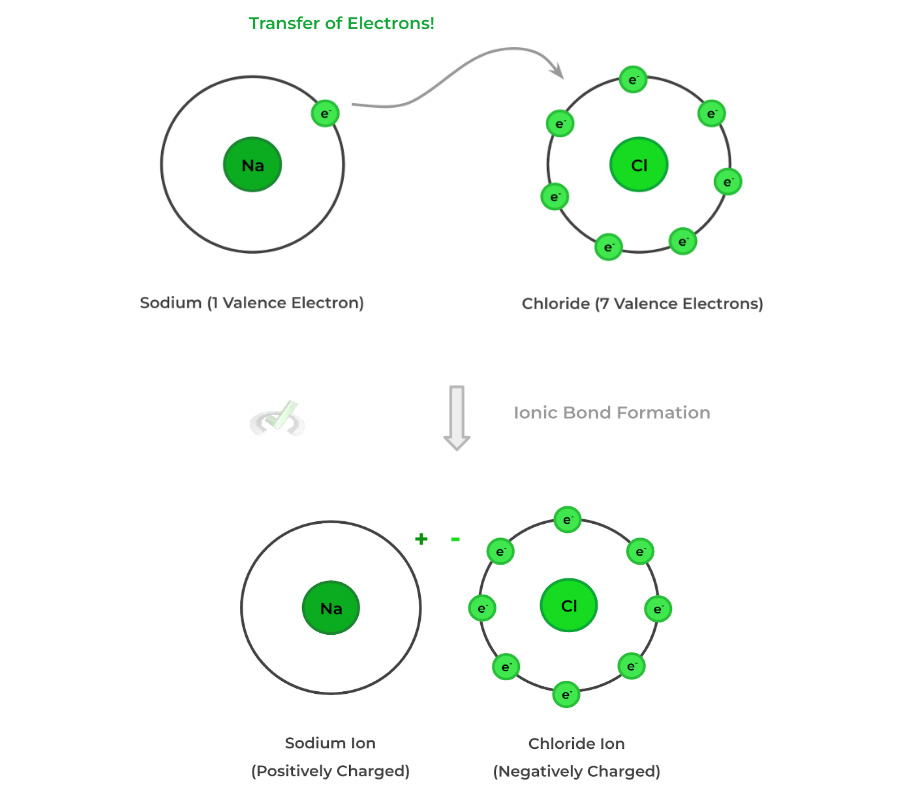
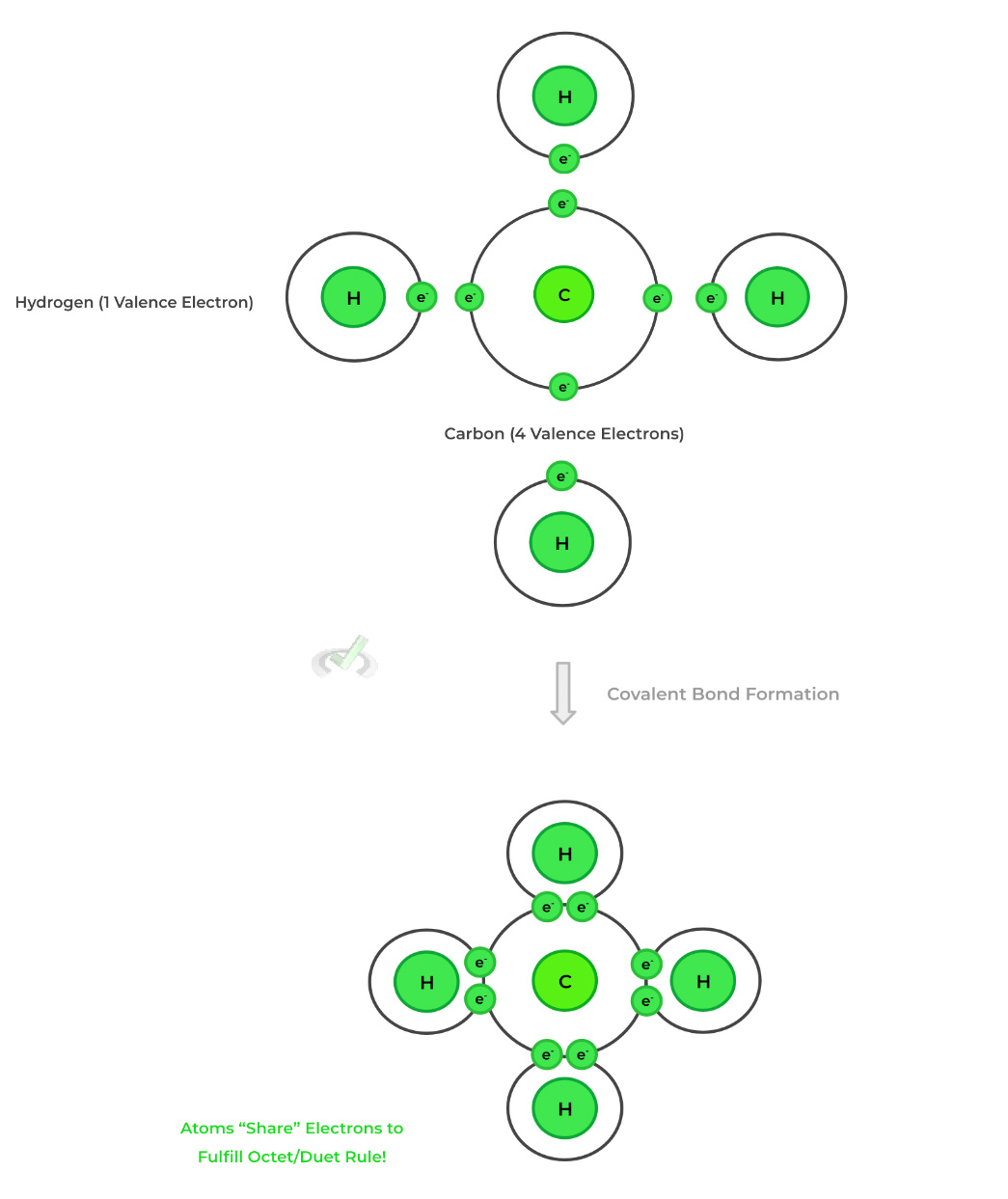
There are 2 main types of chemical bonds to become familiar with: 1) ionic and 2) covalent bonds. Chemical bonds are formed via how they distribute the electrons on their valence shell — this also explains how each bond differs from one another.
Ionic bonds involve the transfer of electrons from one element's valence shell to another. This results in the formation of ions (charged atoms) which are then attracted and bond with one another via electrostatic interactions.Conversely, covalent bonds involve atoms sharing electrons with one another to form bonds and fulfill the octet/duet rule (more on that in the next section).
Because of the electrostatic attraction that occurs in ionic bonds, it has the higher bonding strength of the 2 types of bonds!
Full Study Notes : Types of Chemical Bonds on the MCAT
For more in-depth content review on the type of chemical bonds!, check out these detailed lesson notes created by top MCAT scorers.
2. The Octet Rule and Basics of Lewis Dot Structures
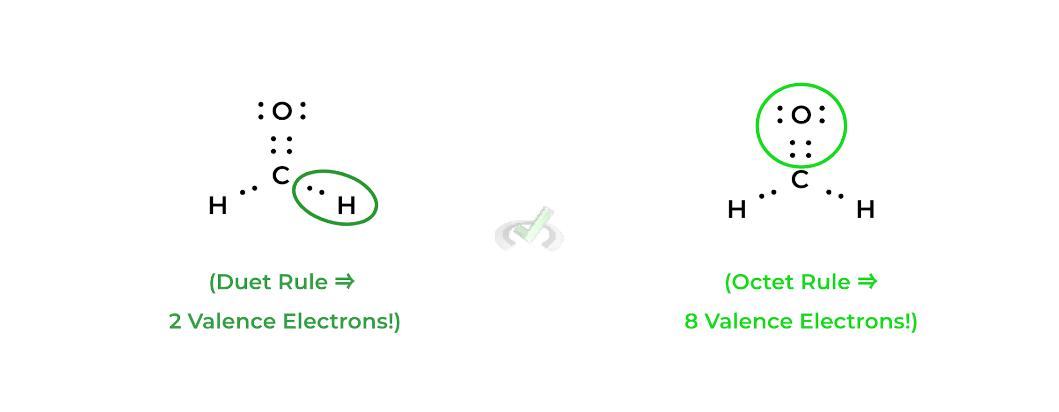
One of the most fundamental rules of thumbs in chemical bond formation is the octet rule which states that chemical bonds will form in a way where the valence shell of all the atoms will have 8 electrons (hence the term “octet”).
However, as with all rules, there are always exceptions: this rule mainly applies to the s and p block elements (i.e. the main group elements). Molecules that have transition metals often don’t follow this group as well as peroxides.
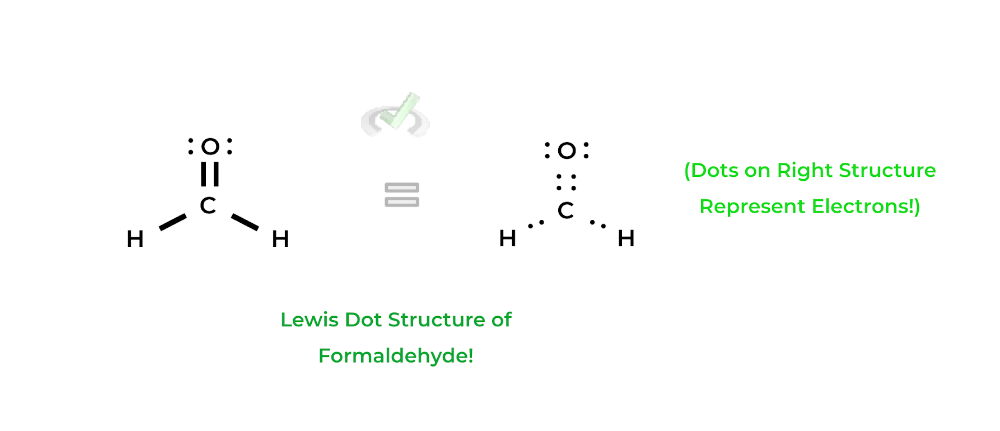
There’s also the duet rule which only applies hydrogen and helium and states that these elements want to have 2 electrons in their valence shell: together, both of these rules help us to build Lewis structures. Let’s take a look at formaldehyde!In this case, the bonds in formaldehyde (represented by a straight line) are all covalent bonds! As shown above, each straight line represents 2 electrons which in this case are shared via the covalent bonds — thus, they’re called bonding electrons.
Likewise, the dots on top of the oxygen atom are called nonbonding electrons, as they don’t participate in bonding interactions: these electrons are also known as “free” electrons as they remain free in the orbitals not participating in bonding.
Finally, notice how the lewis structure of formaldehyde fulfills both the octet rule (for carbon and oxygen) and the duet rule (for the hydrogens). Also, recall that because the bonds are covalent bonds, the atoms share their electrons.Luckily, you won’t have to draw the Lewis structures as they’ll be provided for you in the answer choices and passages! However, it’ll be your job to determine which Lewis structure is the correct one given answer choices!
Full Study Notes : The Octet Rule and Basics of Lewis Dot Structures on the MCAT
For more in-depth content review on Lewis structure and the octet/duet rule, check out these detailed lesson notes created by top MCAT scorers.
3. Importance of Formal Charges
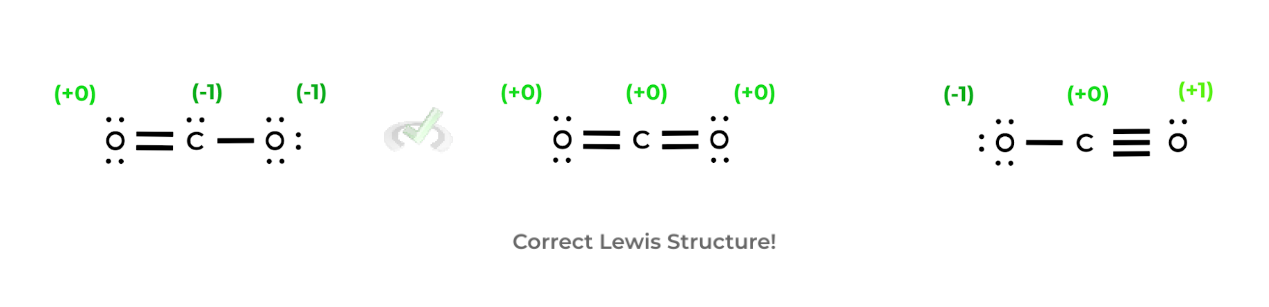
As we pointed to in the previous article, it’ll be your job on the MCAT to determine the most likely Lewis structure of a molecule. Here, we can use formal charges — which is a way to assign hypothetical charges on the molecules — to help us to determine which Lewis structure is the most correct! Let’s look at an example below!
As shown above, the correct Lewis structure for carbon dioxide is the one in the middle! We know this via the assignment of the formal charges on the atoms! To find the most correct Lewis structure via the formal charges, follow the general rules below:
A. The sum of the formal charges should always equal the charge of the molecule.
- If the molecule is neutral, such as carbon dioxide, all the charges will add up to +0.
- If the molecule is an ion, such as a phosphate ion, the charges will add up to -3.
B. Try, first and foremost, to have all the formal charges of the atoms be +0.
- If this can’t be done, construct the Lewis structure to minimize the formal charges that are not +0 and that the formal charges as “close to +0” as possible (i.e. -1 is better than -3).
C. Additionally, try also to put the negative formal charges on the more electronegative atoms (i.e. -1 on oxygen is better than -1 on carbon).
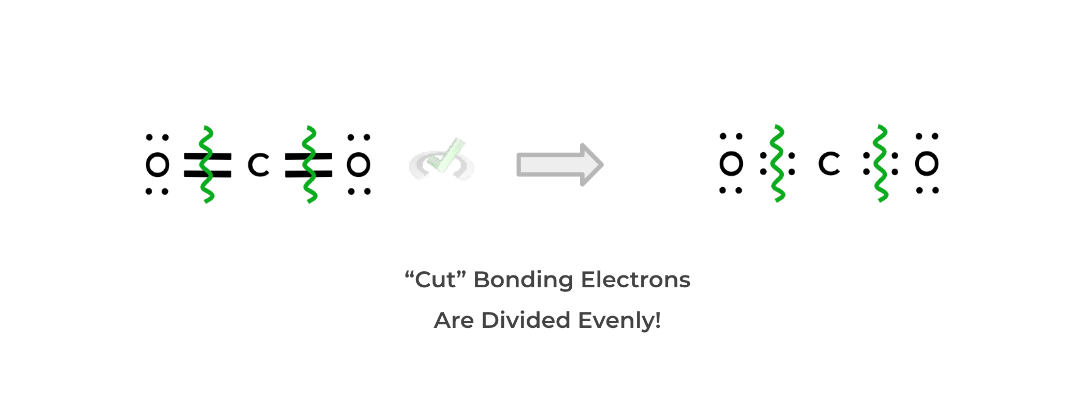
When assigning formal charges, we assume that the electrons' covalent bonds are shared equally between the atoms. In order to calculate the formal charge of an atom in a Lewis structure, follow this trick below using carbon dioxide as an example:
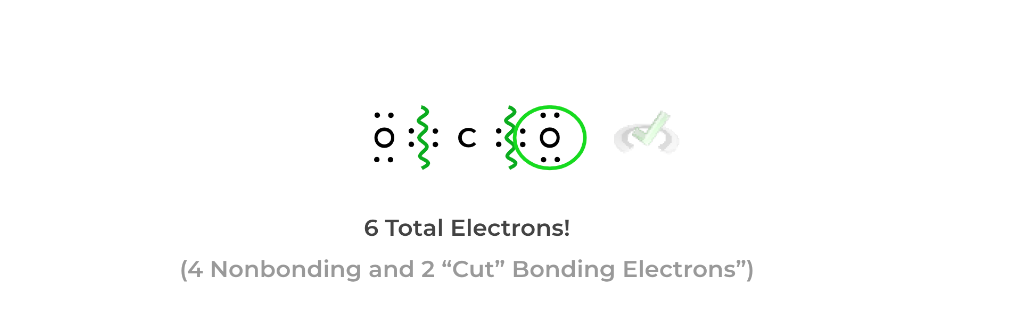
Identify the bonds of the atom of interest and “cut the bonds in half” as shown below. This will evenly distribute the bonding electrons as shown below:Afterwards, count the number of nonbonding electrons and “cut” bonding electrons for the atom. For oxygen, there are 4 nonbonding electrons and 2 “cut” bonding electrons.
Finally, take this number of electrons (we’ll call it the counted electrons) and subtract it from the number of valence electrons on the atom. Oxygen has 6 valence electrons, so we subtract 6 from 6 to get a formal charge of +0.
Contrast this to oxidation states which are also hypothetical charges, but assume an unequal distribution of electrons between atoms and are mostly used for balancing redox reactions!
Full Study Notes : Importance of Formal Charges on the MCAT
For more in-depth content review on the importance of formal charges, check out these detailed lesson notes created by top MCAT scorers.
4. Ionic Bonds
Let’s go a little bit more in detail into the different characteristics of ionic bonds! As we mentioned above, due to the electrostatic interactions of the bond, ionic bonds are the strongest of all the types of chemical bonds!
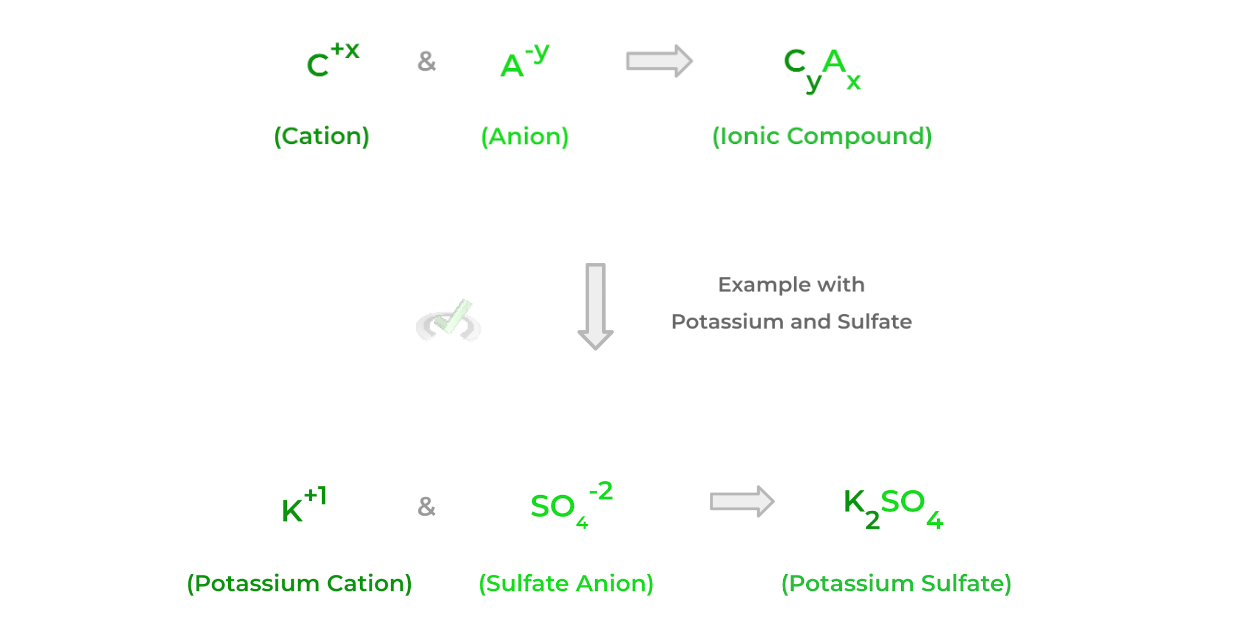
Furthermore, as indicated by the name, ionic bonds are constructed via the ions from the transfer of electrons between atoms! Additionally, if given a cation and anion, we can easily determine the chemical formula of the resulting ionic compound via the equation below:Notice how the subscripts of the atoms in the ionic compounds are basically the charge of the opposite ion. Think of it like a swipe-swap of the subscripts of the ionic charges!
In the above example, potassium has a subscript of 2 because of the -2 charge on the sulfate ion. Likewise, sulfate has a subscript of 1 (not shown because implied) because of the +1 charge on the potassium cation.
Full Study Notes : Ionic Bonds on the MCAT
For more in-depth content review on ionic bonds, check out these detailed lesson notes created by top MCAT scorers.
5. Covalent Bonds
Compared to ionic bonds, covalent bonds have an extra degree of complexity as they deal with the sharing of electrons between atoms. Let’s discuss some of the more important characteristics concerning covalent bonds!
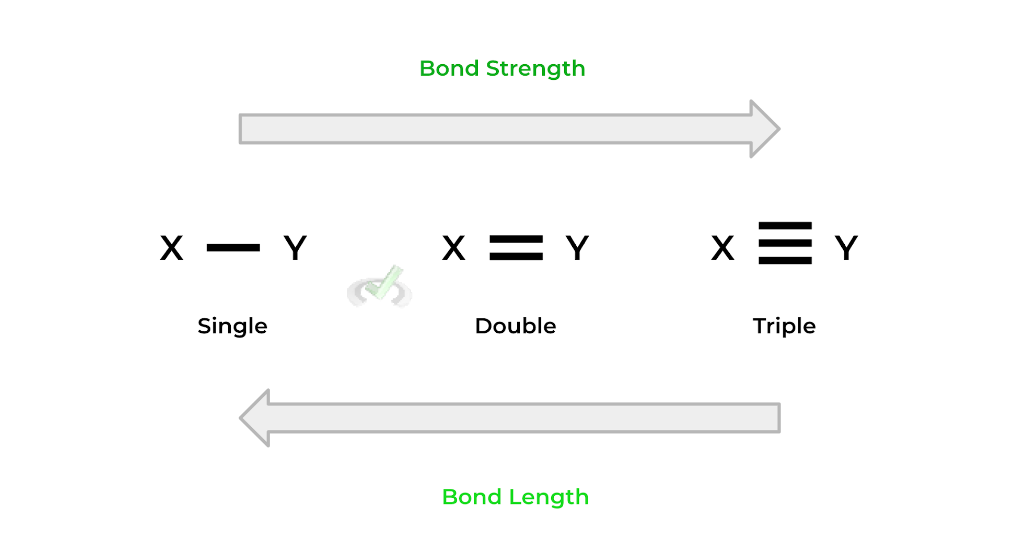
As shown above, with the example of formaldehyde, there can be single, double, and triple covalent bonds between atoms, with each bond still representing the sharing of 2 electrons.
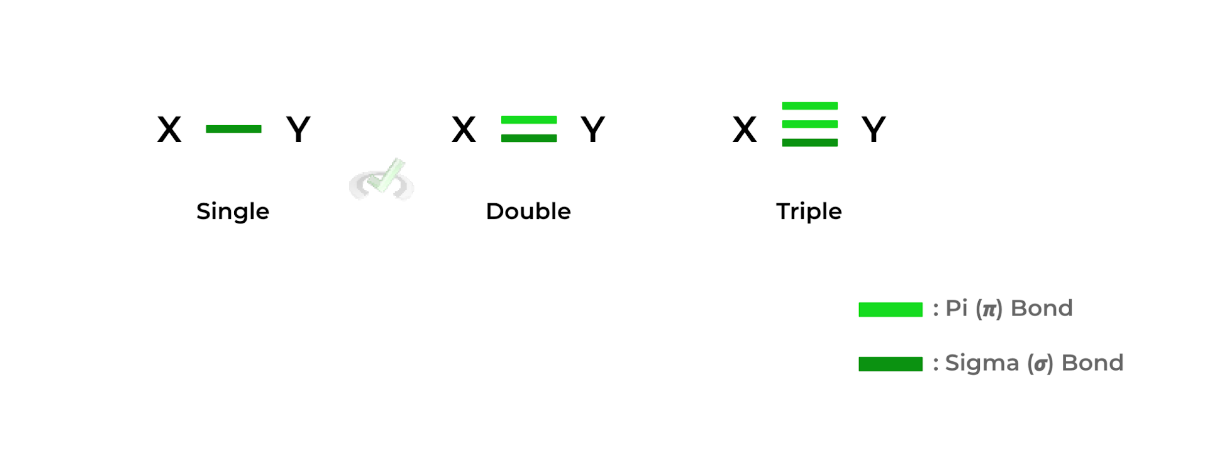
These bonds also differ in their bond strength and length: bond length increases from triple to single bond while bond strength increases from single to triple bond. They’re essentially the reverse of one another!Additionally, these bonds also have specific names or terms: single bonds are also called sigma (𝝈) bonds. Any additional bonds after the single bond are called pi (𝞹) bonds.
For example, the double bond has one sigma bond and one pi bond. Likewise, the triple bond has one sigma bond and two pi bonds
Full Study Notes : Covalent Bonds on the MCAT
For more in-depth content review on main characteristics of covalent bonds, check out these detailed lesson notes created by top MCAT scorers.
6. Molecular and Electronic Geometry
An important aspect of covalent bonding in molecules is that the molecules are theorized to have specific 3D conformation. These are probably the most common shapes that come to mind, such as the ball-and-stick models you’ve maybe seen!
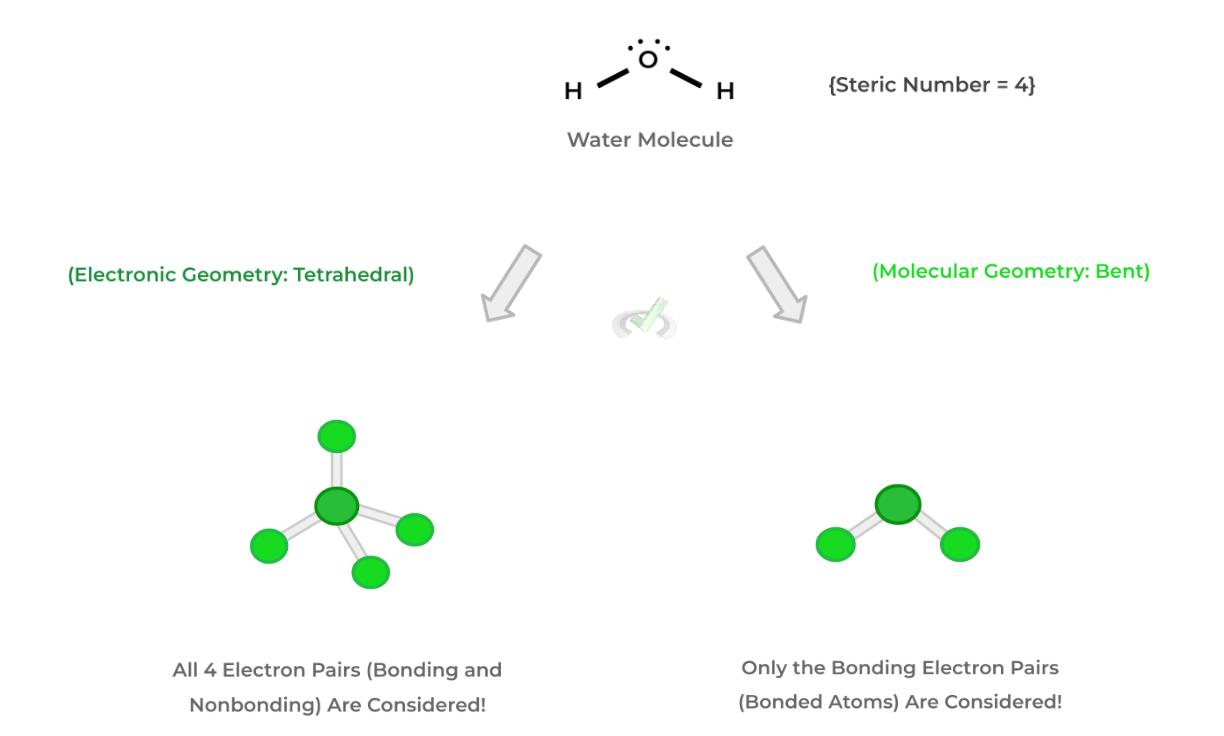
2 important ideas of the 3D shape of molecules are that there’s a main central atom and that the electron pairs — both bonding and nonbonding — will affect the shape of the molecule due to electron repulsion.
The main difference between electron and molecular geometry is which electron pairs are considered: electron geometry considers the arrangement of all the electron pairs (bonding and nonbonding), while molecular geometry considers only the arrangement of the bonding electrons. Look at the example below!The electronic geometry considers all the electron pairs into its 3D shape: the central atom of oxygen has 2 pairs of nonbonding electrons and 2 pairs of bonding electrons (via the 2 H atoms) and has a tetrahedral geometry.
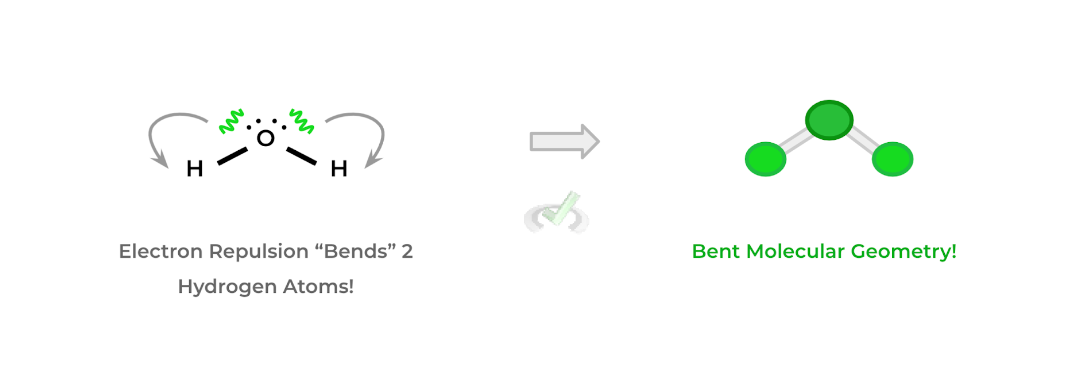
Conversely, molecular geometry considers only the shape of the bonding electron pairs into its 3D shape — however, it still considers how the electron repulsion of the nonbonding electron pairs.
In this case, the 2 H atoms (which are the bonding electron pairs) are pushed closer together as a result of the repulsion of the electron pairs on the oxygen atom which results in a bent geometry.Full Study Notes : Molecular and Electronic Geometry on the MCAT
For more in-depth content review on the molecular geometry won’t always be the same as the electronic geometry, check out these detailed lesson notes created by top MCAT scorers.
7. Types of Intermolecular Interactions
An important aspect that you’ll have to be comfortable with come to the MCAT test date is to understand the different interactions between molecules — hence, the term intermolecular interactions.
There are 3 main intermolecular interactions we’ll discuss: Van der Waals forces, dipole-dipole interactions, and hydrogen bonding, with each having its own caveats and unique characteristics!
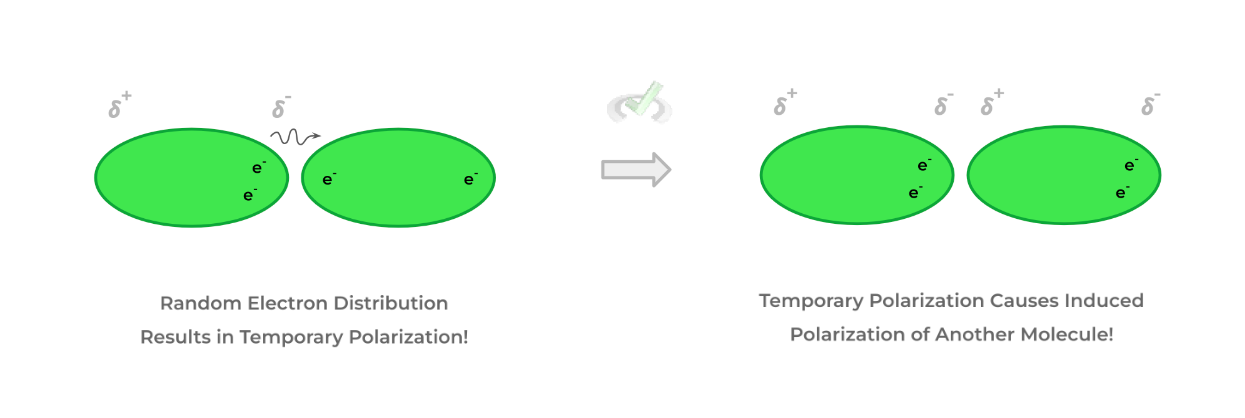
A. Van der Waals Forces
- These are the weakest of the intermolecular attractive interactions because they rely on “weak” dipole-dipole interactions.
- This occurs when there’s a temporary polarization within molecules due to the random distribution of electrons which can generate a temporary dipole. Consequently, this temporary polarization can cause an induced dipole to another molecule, as shown below!
- This is the only intermolecular interaction that hydrophobic, nonpolar molecules participate in!
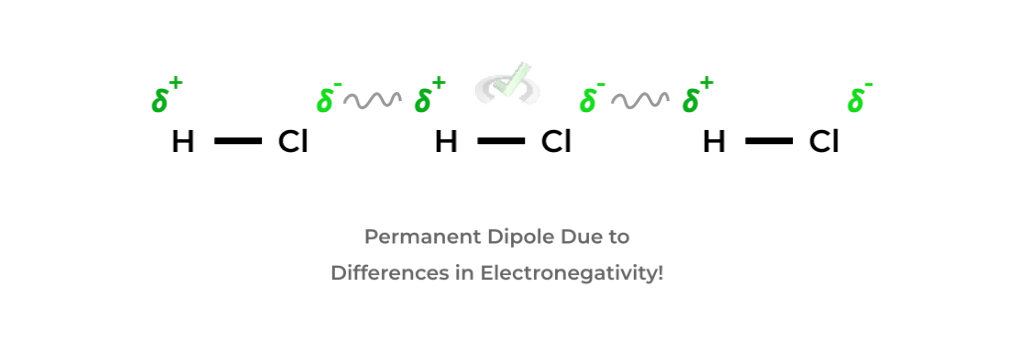
B. Dipole-Dipole Interactions
- These interactions are due to differences in the distribution of electrons within a molecule! In molecules, a more electronegative atom will have a higher electron density!
- As such, this creates a dipole where the more electronegative atom has a negative dipole and the other attached atom has a positive dipole — with many of these interactions, these molecular dipoles interact in the way shown below!- As noted above, the differences in electronegativity result in a permanent dipole as opposed to the temporary dipole in Van der Waals interactions which is why this interaction is much stronger!
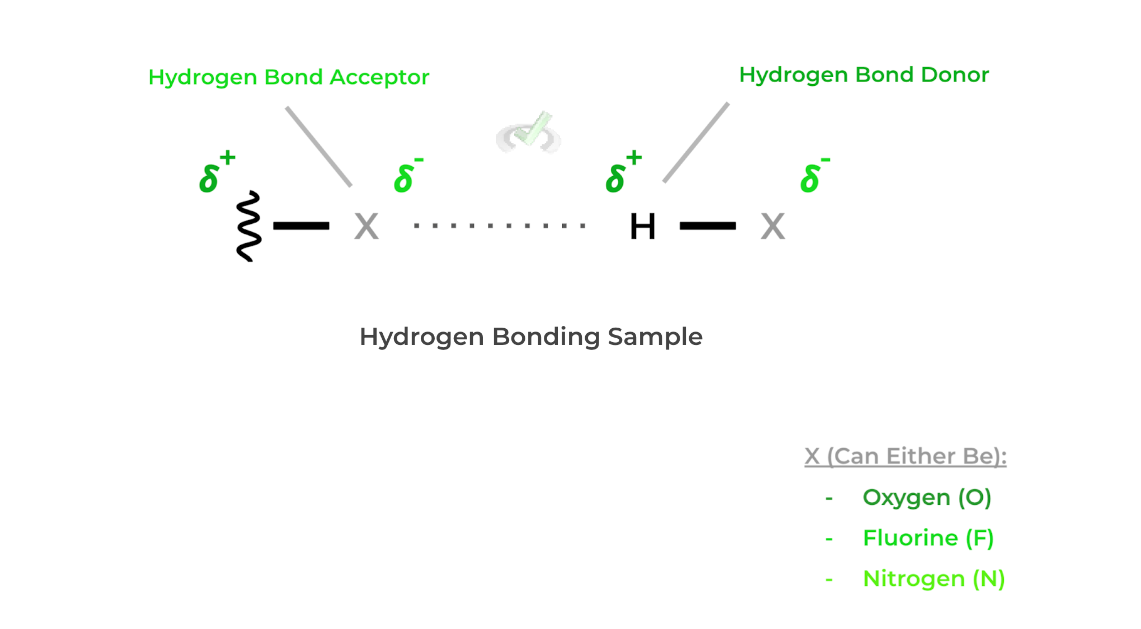
C. Hydrogen Bonding
- Out of all the three intermolecular interactions we talked about, this is the strongest. A great way to think about this intermolecular interaction is that it’s essentially a stronger type of dipole-dipole interaction!
- Hydrogen bonding involves a hydrogen bond donor (an electronegative atom with a hydrogen atom) and a hydrogen bond acceptor (another electronegative atom) as shown below!- As shown above, hydrogen bonds can only occur with oxygen (O), fluorine (F), and nitrogen (N) for the scope on the MCAT!
Full Study Notes : Types of Intermolecular Interactions on the MCAT
For more in-depth content review on different types of intermolecular interactions, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about chemical bonds and interactions in general chemistry!
Term | Definition |
|---|---|
Ionic Bond | A type of bond formed via the transfer of electrons; The strongest type of chemical bond due to the electrostatic attraction between the cation and anion |
Covalent Bond | A type of bond formed via the sharing of electrons; Atoms will form covalent bonds usually the fulfill the octet/duet rule |
Octet Rule | General rule of thumb for most s and p block elements stating that these elements prefer to have 8 electrons in their valence shell |
Duet Rule | General rule of thumb for hydrogen and helium stating that these elements prefer to have 2 electrons in their valence shell |
Formal Charge | A type of way to assign hypothetical charges to atoms in a molecule to determine the most correct Lewis structure; Assumes equal sharing of electrons |
Molecular Geometry | 3D shape of molecule which takes into account only how the electron bonding pairs (those bonded to atoms) are configured |
Electronic Geometry | 3D shape of a molecule which takes into account all types of electron pairs (both bonding and nonbonding) |
Van der Waals Forces | A type of intermolecular interaction which involves the attraction between molecules due to temporary, induced dipoles |
Dipole-Dipole Interactions | A type of intermolecular interaction which involves the attraction between molecules due to permanent molecular dipoles |
Hydrogen Bonding | A type of intermolecular interaction which is basically a stronger type of dipole-dipole interaction; Oxygen, fluorine, and nitrogen are the only electronegative atoms involved in hydrogen bonding |
Additional FAQs - Bonding and Chemical Interactions on the MCAT
What is a Covalent Bond? – MCAT
How Do You Memorize Chemical Bonds? – MCAT
What are the 4 Types of Chemical Bonds? – MCAT
What is a Coordinate Bond? – MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Acids and Bases on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT








 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot