I. What are Cellular Membrane Dynamics?
In another article, we made the analogy to how the membrane is like the cell’s version of the Great Wall of China! However, it’d be a little deceiving to think of the cell membrane as being static and consistent throughout the cell’s lifetime!
In fact, the cell membrane is in a constant state of change and development, with the movement of phospholipids, the ongoing addition and removal of membrane proteins, like the Great Wall went through constant reconstruction and remodeling.
Because of its continuous development, the cell membrane remains one of the most flexible and adaptable organelles within the cell! Let’s go ahead and dive into all the main concepts you’ll need to know for membrane dynamics for the MCAT!
II. Membrane Dynamics and General Function in Cell Containment
The fluid mosaic model is an important framework when discussing the structure and function of the cell membrane. Let’s review it a bit more below!
A. Fluid Mosaic Model and Membrane Fluidity
This model basically reiterates how we’ve described the cell membrane: a semipermeable phospholipid bilayer embedded with proteins, carbohydrates, and other molecules. We’ll look more to the fluidity component of the membrane in this article!
To maintain a flexible nature, the phospholipids don’t just remain in one place in the membrane but are in constant motion! There are 2 types of phospholipid membrane movements: 1) intramembrane and 2) intermembrane.
I. Intramembrane (One Membrane)
This refers to the movement of phospholipids LATERALLY within one membrane! Additionally, this type of movement occurs rapidly and is ATP independent! (i.e. energy not required)
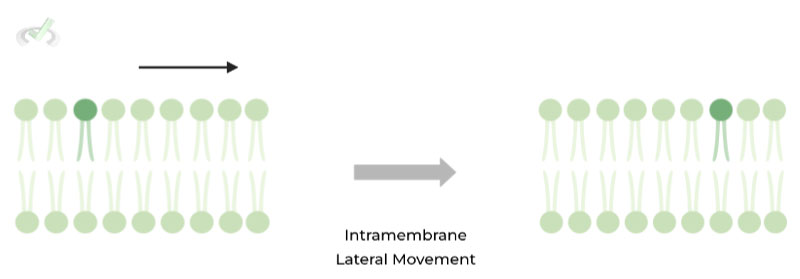
II. Intermembrane (Two Membranes)
In intermembrane movement, phospholipids move BETWEEN the two membranes; however, this type of movement is slower and sometimes ATP dependent.
This is due to the unfavorable interaction that occurs when the polar phosphate head interacts with the hydrophobic core during movement between membranes. As such, enzymes are embedded in the membrane to catalyze this movement:
- Flippases: Movement from outside to inside layer – ATP Dependent
- Floppases: Movement from inside to outside layer – ATP Dependent
- Scramblases: Swaps 2 phospholipids (one inner & outer) – ATP Independent
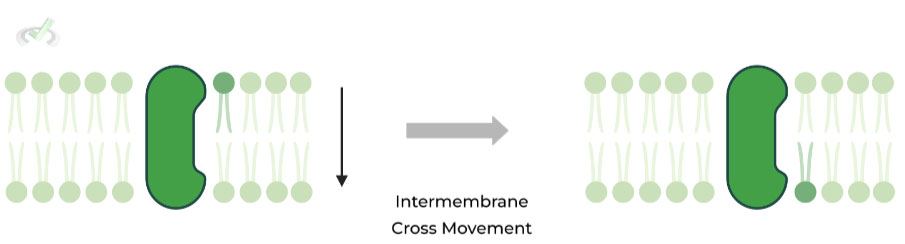
To make an analogy, imagine a concert venue where the sections are divided by fences. Like intramembrane movement, you would be able to move side to side in one section, passing my neighboring concertgoers!
However, if you wanted to move closer or farther away from the stage, you would have to hop over those fences which requires a bit more work like intermembrane transport!
B. Semipermeability
You probably saw that we added an additional term to describe the cell membrane via the fluid mosaic model: semipermeable. As the term implies, the cell membrane is only permeable to particular external particles and agents when allowing what enters into the cell.
While the semipermeable nature is often associated with proteins (i.e. transport proteins), it can also be attributed to the membrane itself!
Because of the fatty acid core, hydrophobic, nonpolar substances can cross the membrane easily. Conversely, polar substances will have a much harder time crossing the membrane and require transport proteins. Look to the diagram below!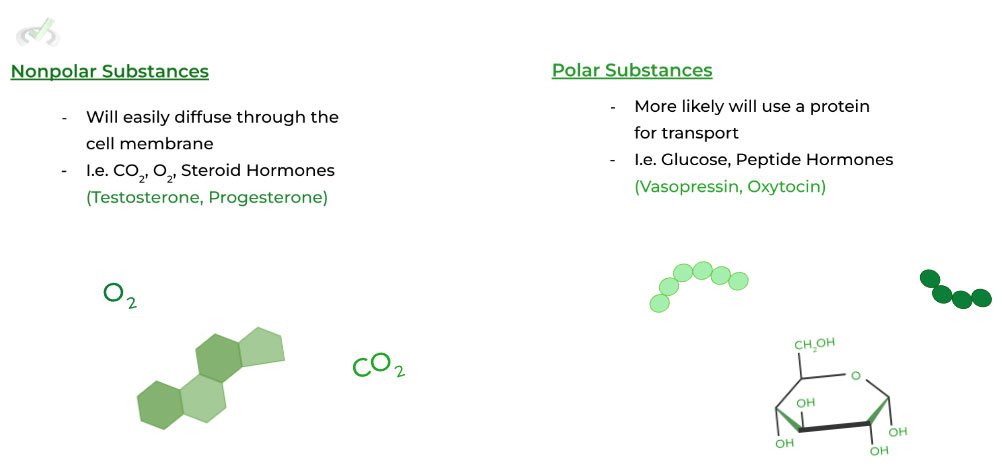
Size also plays a role in determining how easily a substance can pass through the membrane. The smaller the object, the easier it can cross the membrane; the larger the substance, the harder it is to cross.
C. Fatty Acid Saturation & Cholesterol: Role in Membrane Fluidity
In addition, the saturation of fatty acids and cholesterol play a significant role in influencing membrane fluidity. Let’s take a look at their roles below!
I. Fatty Acid Saturation
Recall that fatty acids can either be saturated or unsaturated: saturated refers to when the fatty acid’s hydrocarbon chain DOES NOT CONTAIN a double bond whereas unsaturated refers to when the hydrocarbon chain DOES CONTAIN a double bond.
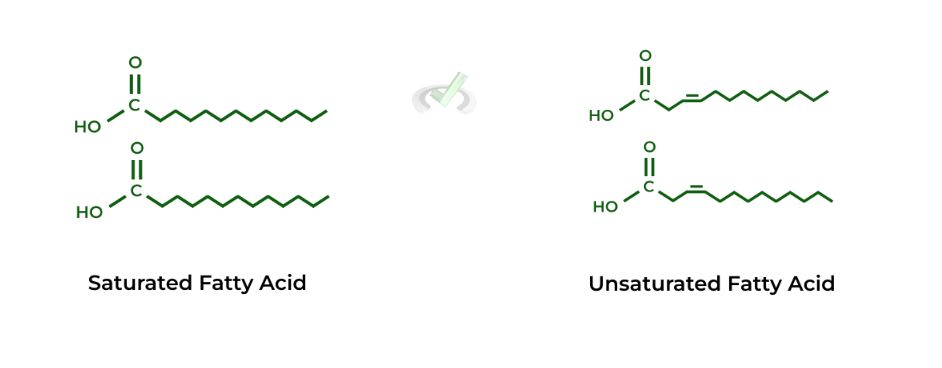
The double bond in unsaturated fatty acids induces a “kink” or a bend within the chain, which prevents the phospholipids fatty acid tails from stacking together, thus INCREASING membrane fluidity.
Because there is no double bond and no kink within saturated fatty acids, the fatty acids can interact and stack together much better, REDUCING membrane fluidity.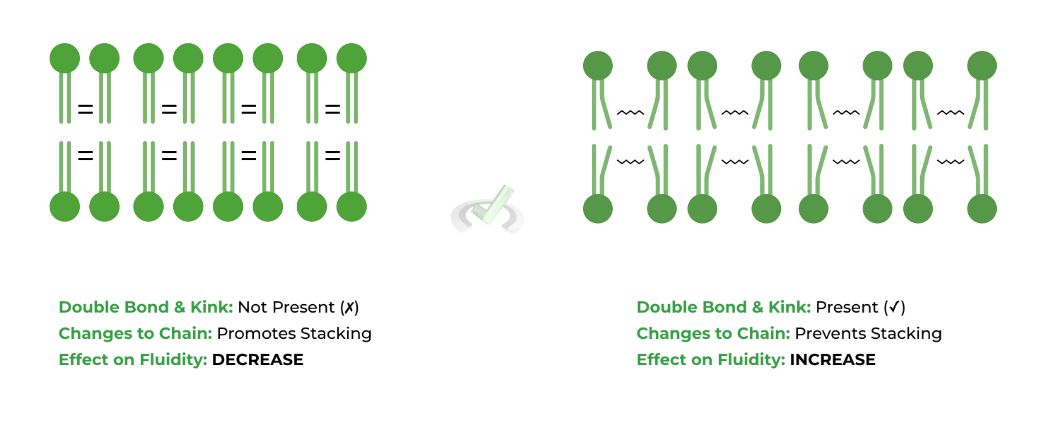
The ratio and proportion of saturated and unsaturated fatty acids within the membrane most often depends on the environmental conditions a cell is in, specifically the temperature.
High temperatures increase fluidity causing the phospholipids to separate while low temperatures decrease fluidity causing the membrane to become static – this comes into play when discussing the optimal saturated & unsaturated FA ratios in the cell membrane.
II. Cholesterol
Cholesterol has a dual function in maintaining membrane fluidity where in high temperatures, it aids to decrease membrane fluidity and in low temperatures, it increases membrane fluidity.
This is due to its large, hydrophobic ring structure. In high temperatures, the ring structure forms strong hydrophobic interactions with the fatty acid tails to “pull” them closer together, DECREASING fluidity.
In low temperature, when the fatty acids close in, the cholesterol’s large ring structure prevents them from stacking together, acting like a sterical hindrance INCREASING fluidity.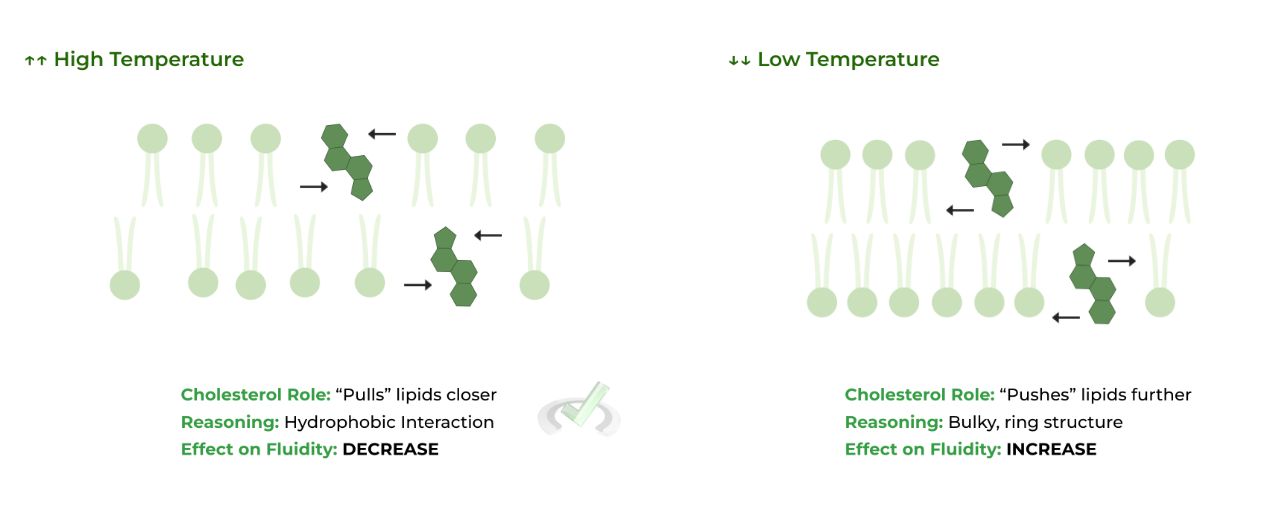
Here’s another analogy! Picture 1 big balloon tied to 2 smaller balloons on the side. When the smaller balloons drift apart, the strings attached on the big balloons prevent them from drifting too far, just like cholesterol in high temperatures.
When the smaller balloons start to close in, the bulk of the large balloon prevents them from coming too close together, similar to cholesterol in low temperatures.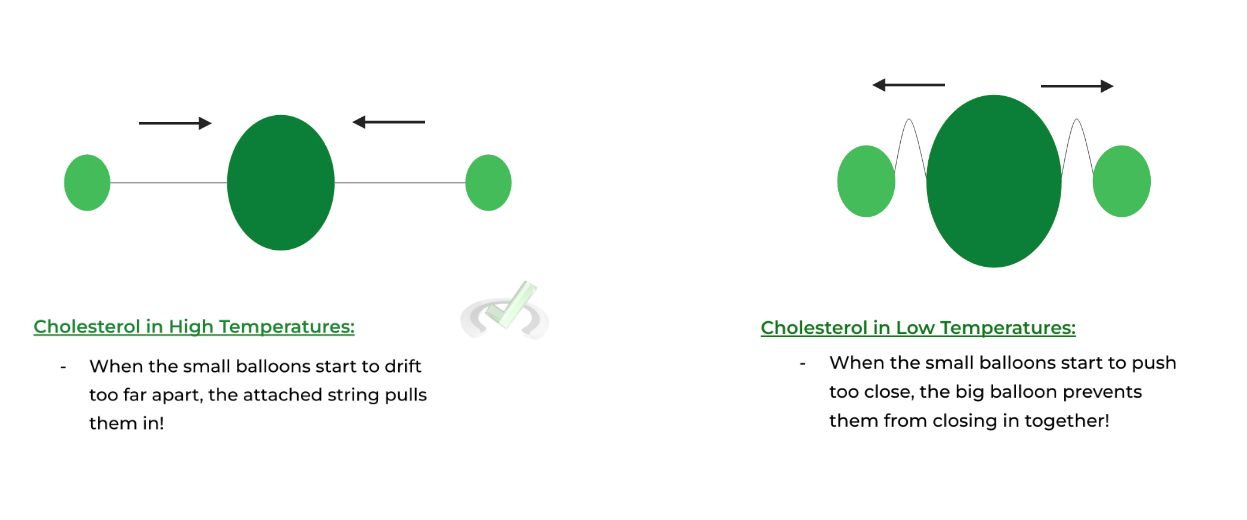
III. Bridge/Overlap
In order to get a better understanding of why substances like CO2 and O2 are nonpolar & can easily cross the membrane, we can look back to dipole vectors and their molecular geometry!
I. Dipoles and Molecular Geometry
Dipoles require that the atoms differ in electron affinity: one high and one low: the high electron affinity atom generates a localized negative charge by “pulling the electrons” towards it, leaving a localized positive charge on the low electron affinity atom.
Dipoles are also affected by the vectors they generate: this is where molecular geometry comes into play, as though a molecule may generate dipole moments, the directional vectors of the dipole moments can cancel each other out, as shown below: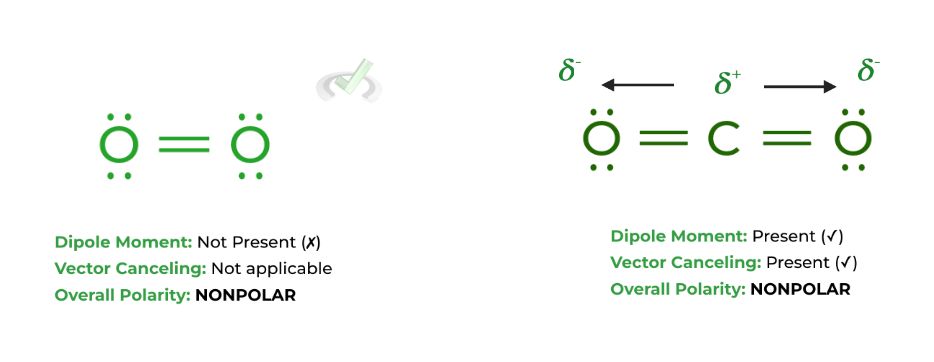
Though carbon dioxide does have dipole moments, the dipoles’ directional vectors cancel each other out as they point in opposite directions, thus making the overall molecule NONPOLAR.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Fluid Mosaic Model and Membrane Fluidity
Describes the cell membrane as a dynamically moving, semipermeable phospholipid bilayer embedded with proteins, carbohydrates, and various other molecules.
The phospholipids actually have the capability of moving within the membrane through 1) intramembrane (one membrane) or 2) intermembrane (two membrane).
Intramembrane describes lateral movement of phospholipids while intermembrane refers to the swapping of phospholipids between layers, which usually requires enzymatic aid.B. Semipermeability
Allows for the strict regulation of what particles/agents are allowed entry to the cell. While usually associated with proteins, the cell membrane itself is also a mechanism in regulating what enters into the cell.
Nonpolar molecules have a much easier time crossing the membrane because of the favorable interactions that occur within the hydrophobic membrane core. Polar molecules have a much harder time crossing and usually require the use of transport proteins.
C. Fatty Acid Saturation and Cholesterol’s Role in Membrane Fluidity
In both cases, the hydrophobic interactions that take place within the hydrophobic core drives their roles in regulating membrane fluidity.
I. Fatty Acid Saturation
Refer to the lack of presence or presence of a double within the long hydrocarbon chain, respectively. The relationship can be summarized below:
II. Cholesterol
Has dual function in regulation membrane fluidity depending on the temperature, also summarized below:
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
The side chain of which of the following amino acids would cross the cell membrane the easiest?
A. W
B. L
C. A
D. I
Ans. C
Looking at all the amino acids (tryptophan, leucine, alanine, and isoleucine), we see that they are all nonpolar – that makes our job easy! Now, we just have to consider the size: alanine has the smallest, nonpolar side chain out of all the amino acids, making it the most likely to cross the easiest.
Sample Practice Question 2
Certain archaea microorganisms, termed extremophiles, can live near hydrothermal vents, where the temperature becomes drastically high! Given this, what would be the most optimal saturated/unsaturated FAs proportion in the cell membrane for these creatures?
A. Low Unsaturated; High Saturated
B. High Unsaturated; Low Saturated
C. Low Unsaturated; Low Saturated
D. High Unsaturated; High Saturated
Ans. A
Because the microorganisms live in areas of such high temperature, the high temperature will cause an increase in membrane fluidity – to account for this, they would need to decrease membrane fluidity.
A decrease in the amount of unsaturated FAs would decrease the amount of kinks and thus would promote the stacking of FAs, DECREASING membrane fluidity.
Likewise, an increase in the amount of saturated FAs would also promote the stacking of FAs, also DECREASING membrane fluidity!







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
