I. What is the Citric Acid Cycle?
If you’ve been on premed (and even med school!) meme pages, you’ve definitely seen the running jokes that come along with this topic, haha! Med students often poke fun at this topic, as this is like the 4th or 5th time relearning this process, lol!
All jokes aside, this is another crucial process that occurs within the body and as such deserves a decent amount of attention when undergoing MCAT prep! It’s definitely helpful if you’ve had a biochem class or 2 under your belt, but we’ll try to make learning this as simple as possible!
Just like the other carbohydrate metabolic processes, we’ll peel back layer by layer! We’ll start off with a general overview then get into the more high yield steps that you’ll most likely encounter. For a more detailed understanding, consult other resources like online textbooks and tutorials!
II. Reactions, Substrates, and Products of the Citric Acid Cycle
Getting a little hint of deja vu, but let’s again start with a general overview definition and diagram of the citric acid cycle!
A. Net Molecular and Energetic Results of Citric Acid Cycle
Also called the Krebs or tricarboxylic acid cycle, the citric acid cycle is a repeating process where an incoming acetyl-CoA is used to synthesize citrate which is then oxidized to generate NADH, FADH2, and GTP.
B. Electron Carrier Producing Steps
The main function of the citric acid cycle is to generate more high energy, electron carriers (i.e. NADH & FADH2) which can be further oxidized in the electron transport chain. As mentioned before, the stored energy is then utilized to power ATP synthesis.
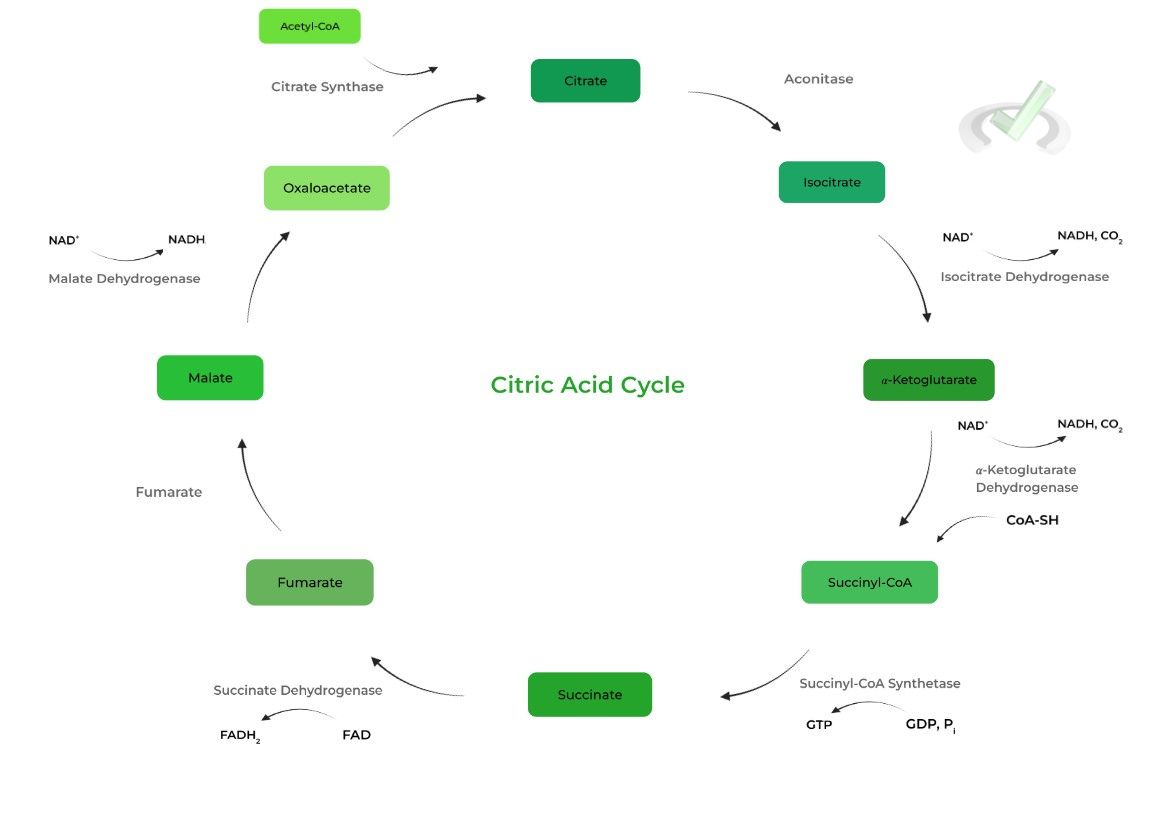
I. Isocitrate to 𝛼-Ketoglutarate (Isocitrate Dehydrogenase)
Analogous to PFK-1 as this is the rate limiting step of the citric acid cycle! As shown below, the reaction produces the first NADH of the process and has CO2 as a by-product.
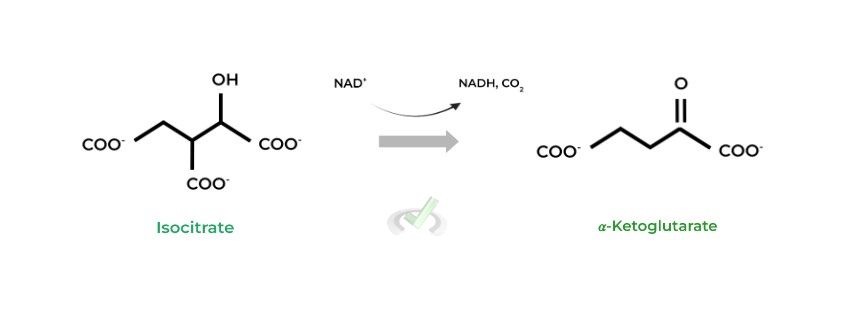
II. 𝛼-Ketoglutarate to Succinyl-CoA (𝛼-Ketoglutarate Dehydrogenase)
Look familiar? That’s because this enzyme is actually a 3 enzyme complex, modeled very similarly to the PDH complex, being why they have the same products (& by-products) of NADH & CO2!
Notice also how an incoming coenzyme A group is also involved in the reaction, generating succinyl-CoA instead of acetyl-CoA as seen in the PDH complex!
III. Malate to Oxaloacetate (Malate Dehydrogenase)
This is the 3rd NADH produced from one round of the citric acid cycle! Additionally, oxaloacetate is regenerated in this step, which can then react with another incoming acetyl-CoA to repeat the process!
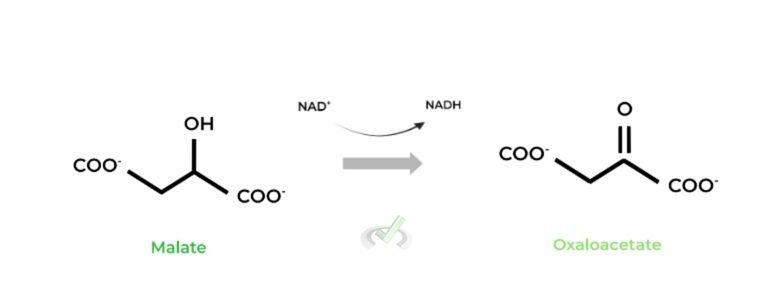
This step really highlights the “cycle” component to the name of the process. The constant regeneration of products, specifically oxaloacetate, allows for this “cycle” process to occur as long as there’s an incoming acetyl-CoA that can react!
IV. Succinate to Fumarate (Succinate Dehydrogenase)
This is the only reaction which produces FADH2 as a high energy electron carrier! It’s also important to note that succinate dehydrogenase has dual function as an enzyme of both the citric acid cycle and the electron transport chain!
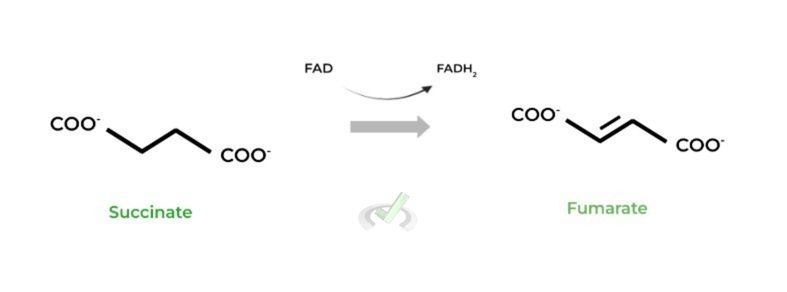
Succinate dehydrogenase is actually embedded within the inner mitochondrial membrane as opposed to the mitochondrial matrix to also contribute to the ETC, which will talk more about later!
C. Other Important Steps
Though the generation of NADH and FADH2 are important, we also want to cover some of the other notable steps of the citric acid cycle!
I. Oxaloacetate to Citrate (Citrate Synthase)
This is where the cycle gets its name! As indicated by the enzyme, oxaloacetate reacts with an incoming acetyl-CoA to form citrate!
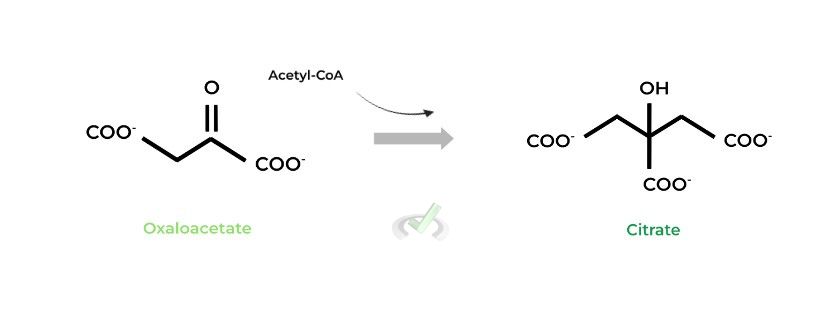
In the reaction, the acetyl group is “merged” with the oxaloacetate, generating a free coenzyme A which can enter another round of the PDH complex.
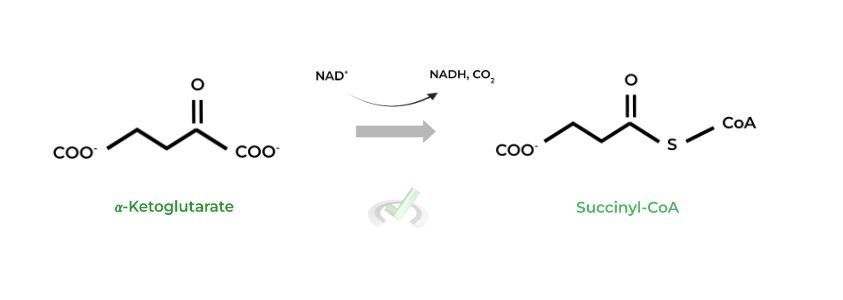
II. Succinyl-CoA to Succinate (Succinyl-CoA Synthetase)
In this reaction, GTP is generated as opposed to the conventional ATP. However, GTP can be converted into ATP via other enzymes; as such, we can view GTP as an ATP equivalent to account for when determining ATP production.
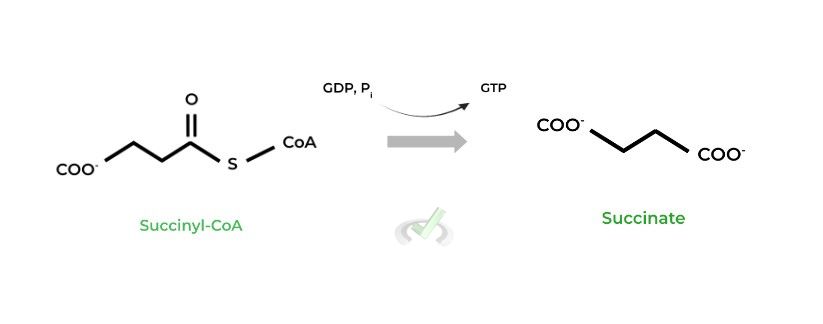
The thioester bond between the succinate and coenzyme group has high energy stored. When this bond is broken, the energy stored is released and is used to power GTP synthesis.
D. Tips about Mnemonics!
There are numerous mnemonics you can search up and use to help memorize the citric acid cycle, but what we recommend is to make some that reflect who you are!
This is a great example of the self-reference effect (talk about a Psych/Soc overlap) as a memory tool to help you in MCAT prep!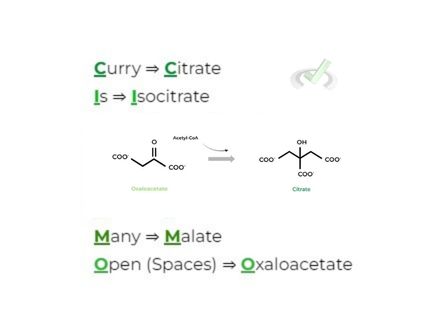
If it isn’t obvious, I’m a huge basketball fan and junkie! Hopefully with the mnemonic above, you can see how I tried to incorporate something I love into a study tool, a strategy that hopefully also works for you all!
III. Bridge/Overlap
Part of the battle of understanding the citric acid enzymes is to categorize them and let their classifications guide your understanding! Let’s briefly review a couple of enzyme classes!
I. Oxidoreductases
As indicated by their name, these enzymes catalyze oxidation-reduction reactions and usually have the name “dehydrogenase”, as with many of the enzymes listed above!
With all the dehydrogenase reactions, the one common concept that occurs with all of them is the transfer of electrons from one molecule to another. In this case, electrons are transferred from the substrate to NAD+ and FAD to reduce them to NADH and FADH2.
II. Synthase v.s Synthetase
There is much debate about how to define these 2 terms; however, after much reading and research, we’ll go with the most agreed upon consensus:
Synthase can synthesize molecules together and DO NOT require an energy source while synthetases DO require an energy source of molecular synthesis.
This is a little tricky for succinyl-CoA synthetase because no common outside source of energy is used, such as ATP. However, recall that the hydrolysis of the thioester bond between succinate and coenzyme A releases a large amount of energy.
While no ATP is being used, the energy is coming from somewhere different; nevertheless, we do have a source of energy that's powering the synthesis of GTP, which is why the enzyme can be categorized as a synthetase.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Net Molecular and Energetic Results of Citric Acid Cycle
Also called the Krebs and tricarboxylic acid cycle; takes in incoming acetyl-CoA and generates citrate to produce high energy electron carriers such as NADH and FADH2.
B. Electron Carrier Producing Steps
These are the steps that contribute to the main goal of producing the high energy electron carriers of NADH and FADH2.
I. Isocitrate to 𝛂-Ketoglutarate (Isocitrate Dehydrogenase)
Analogous to PFK-1 as it’s the rate-limiting step! Generates the first NADH with CO2 as a by-product.
II. 𝛂-Ketoglutarate to Succinyl-CoA (𝛂-Ketoglutarate Dehydrogenase)
Generates the second NADH and CO2 by-product; Has an analogous 3 enzyme complex structure as seen in the PDH complex!
This explains why the products (and by-products) are the same! Note also the analogous involvement of coenzyme A, generating succinyl-CoA here as opposed to acetyl-CoA as seen in the PDH complex.
III. Malate to Oxaloacetate (Malate Dehydrogenase)
Generates the third NADH of the cycle! This is also the last step of the cycle and regenerates oxaloacetate which can react with another incoming acetyl-CoA to generate citrate, thus repeating the cycle.
IV. Succinate to Fumarate (Succinate Dehydrogenase)
Only step of the process which generates FADH2 as an electron carrier; Has dual function as both a citric acid cycle and electron transport chain enzyme!
The enzyme is actually embedded within the inner mitochondrial membrane so as to also partake in the ETC!C. Other Important Steps
These are other steps aside from the electron carrier producing ones that are also of special interest and worth mentioning!
I. Oxaloacetate to Citrate (Citrate Synthase)
Oxaloacetate reacts with an incoming acetyl-CoA to generate citrate which restarts the cycle and the production of NADH and FADH2.
II. Succinyl-CoA to Succinate (Succinyl-CoA Synthetase)
Generates a GTP, which can be considered an ATP equivalent and thus must be accounted for when counting the numbers of ATP produced.
The energy released when the thioester bond between coenzyme A and succinate powers the synthesis of GTP!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
Citrate is a 6 carbon molecule. After the step catalyzed by 𝛼-ketoglutarate dehydrogenase, how many carbons should be present in succinyl-CoA?
A. 2
B. 3
C. 4
D. 5
Ans. C
After the step catalyzed by 𝛼-ketoglutarate dehydrogenase, there are 2 CO2 molecules released as by-products. These account for the 2 carbons that are taken away from the 6 carbon citrate, leaving 4 carbons remaining on succinyl-CoA.
Sample Practice Question 2:
Which of the following amino acids should be found in more abundance on the surface of succinate dehydrogenase compared to the other citric acid enzymes?
A. Acidic
B. Basic
C. Polar
D. Nonpolar
Ans. D
Recall that because succinate dehydrogenase also functions within the electron transport chain, it is also embedded within the inner mitochondrial membrane.
As such, more nonpolar amino acid residues (i.e. valine, alanine) are expected to be on succinate dehydrogenase’s surface as these surface nonpolar amino acids will have favorable interactions with the hydrophobic, lipid core.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
