Enzymes are biological catalysts. In other words, enzymes speed up the rate of a chemical reaction in the body. For example, a car would be a catalyst for someone trying to get from home to work. Instead of walking, a car can speed up the rate at which someone gets to work.
Enzymes are essential for digestion, liver function, and metabolism; among many other bodily processes.
Now that we know the fundamental function of an enzyme we can dive a little further looking at key terms, definitions, and topics that will be important for the MCAT.
Let’s get started!
Enzymes on the MCAT: What You Need to Know?
Introductory biochemistry accounts for 25% of the Biological and Biochemical Foundations of Living Systems section (Bio/Biochem) and 25% of the Chemical and Physical Foundations of Biological Systems section (Chem/Phys).
Enzymes are one of the most heavily tested topics on the MCAT. In fact, there will be several questions in the biochemistry and, potentially, the chemistry section that will hinge on your understanding of enzymes. Enzyme questions can be asked as stand-alone questions or as a part of a passage.
Luckily for you, this review will give you a concise yet comprehensive review of terminology, graphs, and concepts you are required to understand.
Important Sub-Topics - Enzymes
As mentioned above, enzymes are biological catalysts that are made of protein or RNA molecules. As catalysts, they have several important features. 1) They increase the rate of a reaction by stabilizing the transition state, decreasing the activation energy. 2) Enzymes are never destroyed in a reaction, and can be reused over and over again to catalyze the same reaction. 3) Enzymes are reaction specific. This means that for each chemical reaction only one type of enzyme can be used.
Let’s tackle the important subtopics.
1. Enzyme Structure & Function
Enzymes function to speed up chemical reactions in the body, which is accomplished by stabilizing the transition state. This is done by decreasing the activation energy of the transition state, making product formation faster. Importantly, enzymes only affect the kinetics of the reaction (i.e. the speed or how fast a reaction proceeds), and will not alter how much product is made at equilibrium.
Enzymes have two important sites: (1) Active Site: where the substrate binds to the enzyme, and (2) Allosteric Site: another site on the enzyme which can bind specific ligands that regulate the enzyme's activity (like a switch for the enzyme). The allosteric site can bind ligands that upregulate or downregulate enzymatic activity.
Full Study Notes : Enzyme Structure & Function on the MCAT
For more in-depth content review on enzyme structure & function, check out these detailed lesson notes created by top MCAT scorers.
2. Enzyme Models & Classification
Two enzymatic models exist: (1) The Lock & Key Model, and (2) The Induced Fit Model.
(1) The Lock & Key Model illustrates the enzyme and substrate as perfectly complementary to each other; where the enzyme is the lock and the substrate is the key.
(2)The Induced Fit Model illustrates a more flexible model, where the enzyme and substrate are not necessarily complementary prior to binding. Instead, the enzyme is capable of morphing to accommodate an optimal conformation to the transition state — ultimately decreasing the activation energy for catalysis.
Enzymes on the MCAT can be classified into these following categories:
Category | Description/Notes | Examples |
|---|---|---|
Isomerases | Catalyzes isomerization reactions to create different isomers of molecules | Aconitase Phosphoglucomutase Phosphoglucoisomerase |
Transferases | Catalyzes the transfer of functional groups from one molecule to another molecule | Kinase Peptidyl Transferase Acyl Transferase |
Ligases | Catalyze the combining of 2 molecules to make one bigger molecule | DNA Ligase Succinyl-CoA Synthetase |
Hydrolases | Catalyzes hydrolysis reaction which results in the breaking of bonds | Phosphatases Peptidases Na+/K+ ATPase |
Lyases | Catalyze breaking/cleavage of bonds W/OUT the use of hydrolysis | Aldolase Pectin Lyases |
Oxidoreductases |
Catalyzes oxidation-reduction reactions, which involve the transfer of electrons |
Glyceraldehyde-3-Phosphate Dehydrogenase Isocitrate Dehydrogenase |
The MCAT tends to explain a reaction and ask stand-alone or passage-based questions on what category the enzyme may be.
Full Study Notes : Enzyme Models & Classification on the MCAT
For more in-depth content review on enzyme model & classification, check out these detailed lesson notes created by top MCAT scorers.
3. Enzyme Kinetics
Enzyme kinetics refers to the rate (or how fast) an enzyme catalyzes reactions. How fast an enzyme reacts depends on the amount of enzyme present, how much substrate is present, and presence of any inhibitors.
There are 3 main types of graphs used to depict enzyme kinetics;
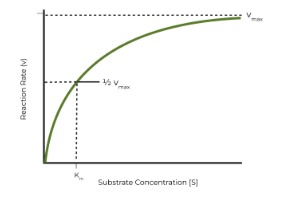
Traditional Hyperbole Graph
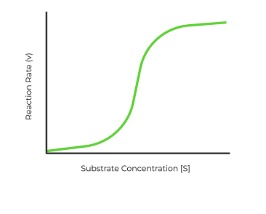
Sigmoidal Graph
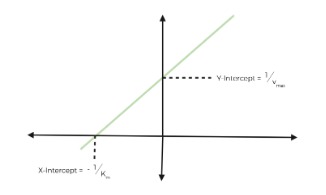
Lineweaver-Burk Plot
Full Study Notes : Enzyme Kinetics on the MCAT
For more in-depth content review on enzyme kinetics, check out these detailed lesson notes created by top MCAT scorers.
4. Enzyme Reversible Inhibition
There are 4 types of reversible inhibitory processes to be familiar with:
For each type of inhibition, you must be familiar with how the inhibitor functions, where the inhibitor binds, how the Km and Vmax change upon inhibition, and recognize how inhibition affects a Linear-Burk Graph.
Full Study Notes : Enzyme Reversible Inhibition on the MCAT
For more in-depth content review on enzyme reversible inhibition, check out these detailed lesson notes created by top MCAT scorers.
5. Enzyme Inhibition: Other Forms
There are three types of other enzymatic inhibitors that are important to know for the MCAT. These include:
Full Study Notes : Enzyme Inhibition: Other Forms on the MCAT
For more in-depth content review on enzyme inhibition other forms, check out these detailed lesson notes created by top MCAT scorers.
Key Terms and Definition — Enzymes
Term | Definition |
|---|---|
Enzyme | Biological catalysts |
Transition State | The highest energy structure along a reaction coordinate for every step of a chemical reaction from reactants(s) to product(s). |
Maximum Enzyme Velocity (Vmax) | The reaction rate when an enzyme is fully saturated by substrate |
Catalytic Efficiency | How efficient an enzyme is at converting substrate to product. |
Km | The binding affinity between enzyme and substrate |
Kcat | The turnover number describing how many substrate molecules are transformed into products per unit time by a single enzyme |
Hill’s Coefficient | Denotes the cooperativity of an enzyme:
|
Isomerases | Catalyzes isomerization reactions |
Ligases | Catalyzes the synthesis of a bigger molecule from 2 smaller molecules |
Transferases | Catalyzes the transfer of functional groups between molecules |
Hydrolases | Catalyzes the breakdown of bonds via hydrolysis |
Oxidoreductases | Catalyze oxidation-reduction reactions |
Lyases | Catalyzes the breakdown of bonds not utilizing hydrolysis |
Competitive inhibitors | Binds to the active site of enzymes. Increases Km and doesn’t affect Vmax |
Noncompetitive | Binds to the allosteric site of an enzyme. Doesn’t change Km and decreases Vmax |
Mixed inhibitors | Binds to the allosteric site of an enzyme. Increases or decreases the Km and Vmax |
Uncompetitive inhibitors | Binds to the allosteric site. Decreases the Km and the Vmax |
Additional Reading Links – Study Notes for Enzymes on the MCAT
For more in-depth content review about enzymes on the MCAT, check out these detailed lesson notes created by top MCAT scorers!
Additional Reading: Biochemistry Subjects on the MCAT:
- Biological Membranes on the MCAT
- Carbohydrate Metabolism on the MCAT
- Carbohydrate Structure on the MCAT
- DNA and RNA on the MCAT
- Amino Acids Peptides Proteins on the MCAT
- Lipids and Lipid Metabolism on the MCAT
- Non-Enzymatic Proteins on the MCAT
- Regulation of Metabolism on the MCAT
- Biotechnology on the MCAT
- Bioenergetics on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
