Chemical kinetics sounds like a bad exam question waiting to happen but the essence of it is pretty simple! When we talk about chemical kinetics, we’re basically thinking of how fast a reaction occurs and the stuff that affects how fast this happens. In our previous introductory article on chemical kinetics, we discussed that kinetics and spontaneity are two different concepts.
Kinetics is concerned with how fast a reaction occurs, while spontaneity is concerned with the likelihood of a reaction. In kinetics, we already know that the reaction will occur; we’re only concerned with how fast this reaction happens or with the stuff that may make the reaction fast.
I. Chemical Reaction in a Nutshell
Now, let’s take a step back and think of what happens in a chemical reaction. During a chemical reaction, we’re changing something through the forming or breaking of bonds. Let’s look at the reaction between nitrogen dioxide (NO₂) and carbon monoxide (CO) to form nitrogen monoxide (NO) and carbon dioxide (CO₂).
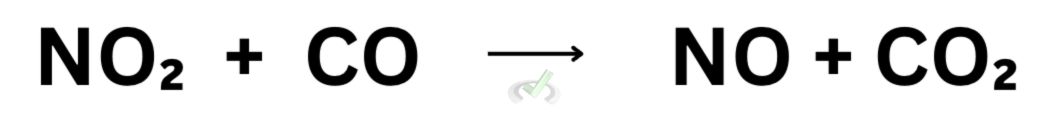
In this reaction, we see the overall reaction of nitrogen dioxide and carbon monoxide. This reaction has a series of steps that lead to the formation of the products, nitrogen monoxide and carbon dioxide.
Imagine you have a jar with a bunch of nitrogen dioxide and carbon monoxide in it. Because we have a large number of nitrogen dioxide, nitrogen dioxide can react with itself to form nitrate and nitrogen monoxide. This counts as the first step of the reaction.
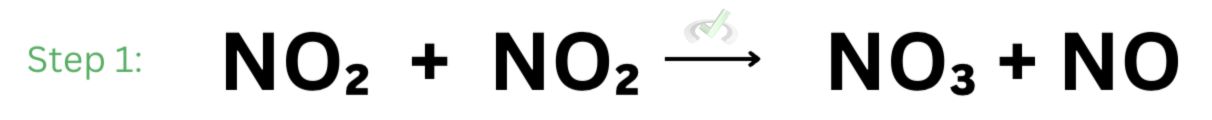
Next, nitrate will react with carbon monoxide to form nitrogen dioxide and carbon dioxide. This is the second step of the reaction.
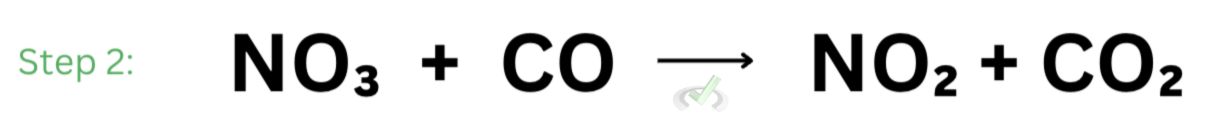
When we add these reactions together, we see nitrogen dioxide and nitrate cancels out. What we end up with is the overall reaction we saw earlier.
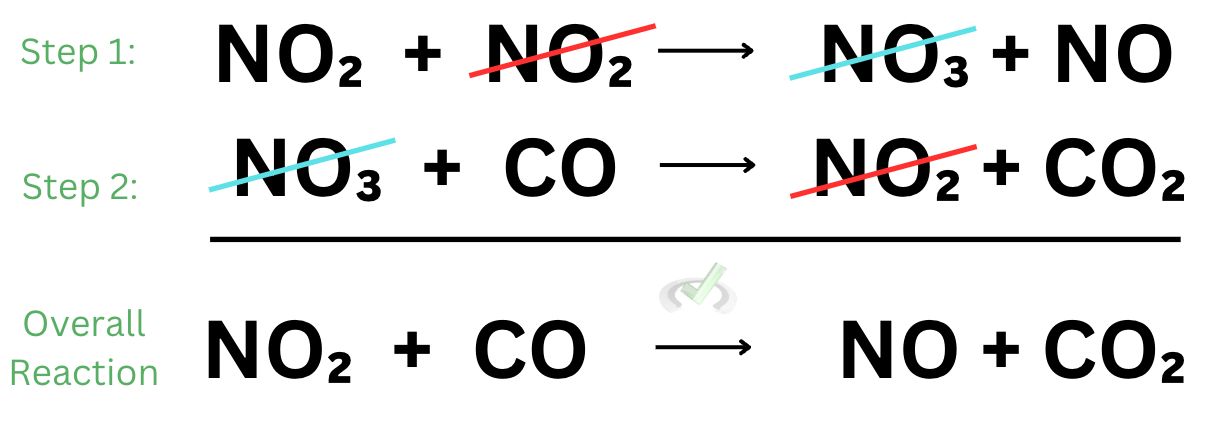
What we see here is that some chemical reactions are not straight forward. In the example above, we see that there are two steps that can happen to form nitrogen monoxide and carbon dioxide. Some reactions have multiple steps to form its original product.
II. Rate Determining Step
In chemical kinetics, we’re interested in how fast a reaction occurs. For a reaction in the previous example, there can be a slow step and a fast step. These two concepts are important because the slow step is what determines the overall rate of reaction.
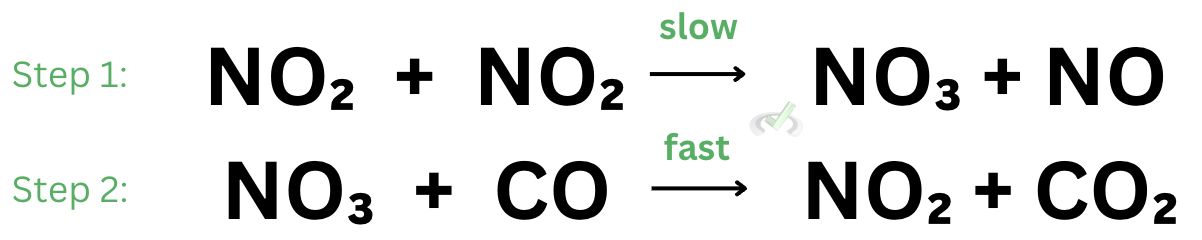
The first step is considered as a slow step because the reaction between nitrogen dioxide requires a high activation energy. Activation energy is simply the energy needed for the reaction to proceed. When a reaction requires high activation energy, the reaction is slow.
The slowest step will determine the overall rate of reaction. Think of it this way: you need to solve a math problem, the most difficult part of solving the problem is actually what sets how fast you’re going to finish that problem.
Here, the slowest step is the rate-determining step. This means that we can find out the overall rate of reaction from the rate of reaction of the slowest step. This will sound complete gibberish, but all you have to take in here is that the slowest step of a multi-step reaction is the rate-determining step of an overall reaction.III. Catalysts and Intermediates
If one odd thing sticks out from the example we’ve used, it’s that we see some chemical species in some steps that do not end up showing up in the overall reaction. These chemical species that do not end up in the overall reaction may be catalysts or intermediates.
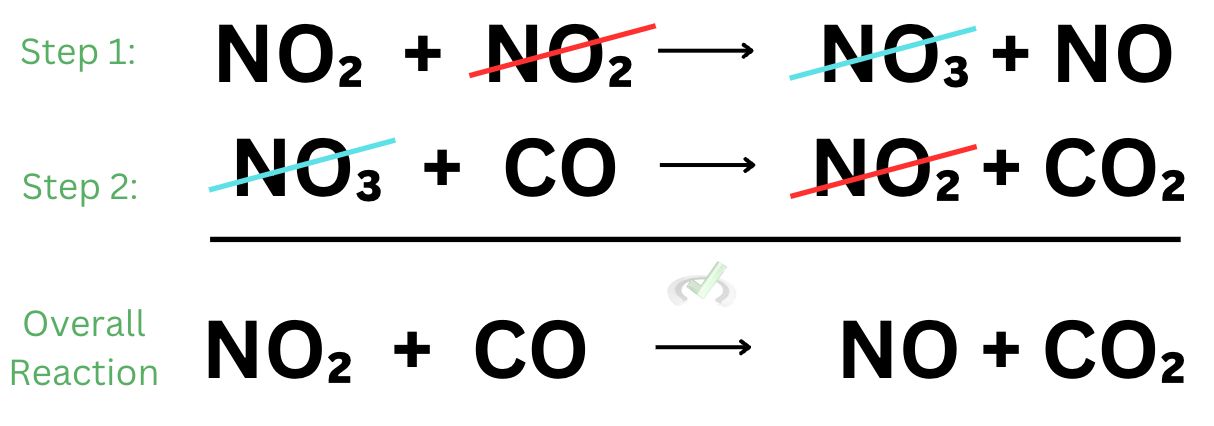
Catalysts and intermediates never show up in the overall reaction. As we see above, they are the ones that get canceled out. Catalysts increase the rate of reaction. They are the species that are consumed first and produced later. When you think about it, a catalyst disappears at first and then shows up later. In the overall reaction, catalysts do not participate because they are consumed and subsequently produced.
In the example above, nitrogen dioxide is the catalyst because while we did use it up in the first step, we still ended up producing it in the second step.
Intermediates are chemical species that are formed in one step of a reaction and consumed in the next. Unlike catalysts that are originally present in the environment, intermediates form as a result of the reaction and they still get consumed in the overall reaction. Intermediate species can be thought of as short-lived chemical species. They merely form as a byproduct and they get immediately consumed to form the product. Intermediates get produced first and gets consumed later.
In the given example, nitrate is the intermediate as it is produced in the first step and eventually consumed in the second step.
IV. Conclusion
Chemical kinetics is concerned with how fast a reaction occurs. As aspiring medical students, you will learn about biochemical processes that rely heavily on enzymatic reactions that explain how enzymes catalyze processes in the body. A chemical reaction can have multiple steps and in order to determine how fast a multi-step reaction occurs, we look at the rate of reaction of the slowest step, also known as the rate-determining step of a chemical reaction. Within these multiple steps of reaction, some chemical species show up or get consumed without being accounted for in the overall reaction. These species can either be catalysts or intermediates.
V. Key Terms
- Catalyst - chemical species that speeds up a chemical reaction.
- Chemical kinetics - a branch of chemistry concerned with the rate of a chemical reaction.
- Intermediate - chemical species formed in one step of a reaction and consumed in the next.
VI. Practice Questions
Refer to the steps of reaction below to answer items 1 and 2.
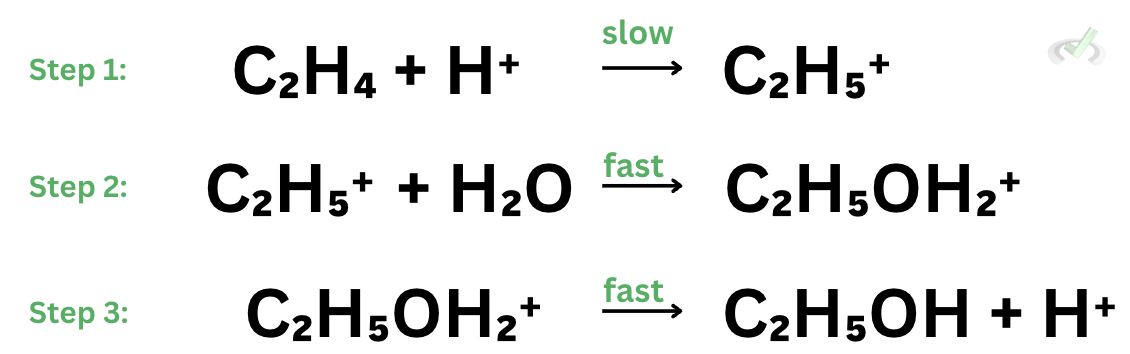
Sample Practice Question 1
Which of the following is the overall chemical reaction?
A. C₂H₅OH₂ → C₂H₅
B. C₂H₄ + H₂O → C₂H₅OH
C. H₂O → C₂H₅
D. C₂H₅OH + H₂O → C₂H₅
Ans. B
Sample Practice Question 2
Which step is the rate-determining step of the reaction?
A. Step 1
B. Step 2
C. Step 3
D. None of the above
Ans. A







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
