There might be questions in chemistry where you are asked about the amount of product produced given a certain amount of reactant. This question will seem like a lot of work, but it’s not! There is absolutely no reason for you to fret since this concept is often one of the easiest problems to solve with the right understanding of stoichiometry
Let’s review stoichiometry.
Stoichiometry is easy. Its only purpose is to identify what we need and what we do not need. For example, if we have 5 moles of carbon and want to know how many grams of carbon this would be equivalent to, we can solve this using stoichiometry.
5 moles = ? grams of carbon
In stoichiometry, we use conversion factors to get the unit we want. The basic principle is that if we don’t want something in the numerator, we simply cancel it by putting the same unit in the denominator. For the example above, we have 5 moles of carbon, and we know that the molar mass of carbon is 12.011 grams per mole.
Since we want to eliminate “moles” and retain only “grams” of carbon, we can multiply the number of moles by their molar mass.

Here, we simply multiplied our moles of carbon by its molar mass. This cancels the units for moles, and we’re left with the equivalent of 5 moles of carbon in grams!
The easiest way to think about it is if we do not want the current unit of a value, we simply multiply it by something with the same unit in the denominator. We do that using relevant conversion factors, which in this case is the molar mass of carbon, 12.011 grams/mole.
I. Calculations of Chemical Equations
During heating, calcium carbonate (CaCO₃) decomposes into calcium oxide (CaO) and carbon dioxide (CO₂). Let’s say we have 402 grams of CaCO3 and we want to know how many grams of CaO will be produced. The chemical equation for this reaction is:
CaCO₃ → CaO + CO₂
Before starting with any problem, the first thing we have to know is the numbers that we’re given. Here, all we know is we have 402 grams of CaCO₃ and we want to know how many grams of CaO will be produced out of this amount.
With this, we can also get the molar mass of CaCO₃ and CaO, which are 100.09 grams/mole and 56.08 grams/mole, respectively.
Remember, in stoichiometry, if we want to remove a unit, we have to place the conversion factor so that it cancels the unit we want to remove.
Here, we start with 402 grams of CaCO₃, and we multiply this by its molar mass. Since we want to remove the unit in grams, we flip the molar mass into 1 mole CaCO₃ over 100.09 grams CaCO₃ so we can cancel grams as we multiply these values.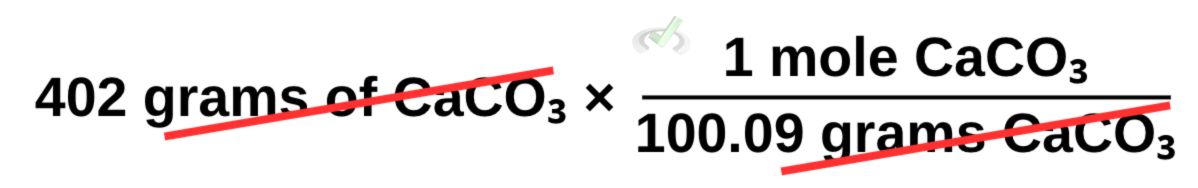
Next, we have to remove the unit in moles of CaCO₃ since, again, what we want to end up with is grams of CaO. We do this by putting the number of moles of CaO that reacts with CaCO₃. We see this by looking at the coefficient of the balanced chemical equation. Here, we see that only 1 mole of CaO and CaCO3 would have to react with each other. We can then say that 1 mole of CaO reacts with 1 mole of CaCO₃.
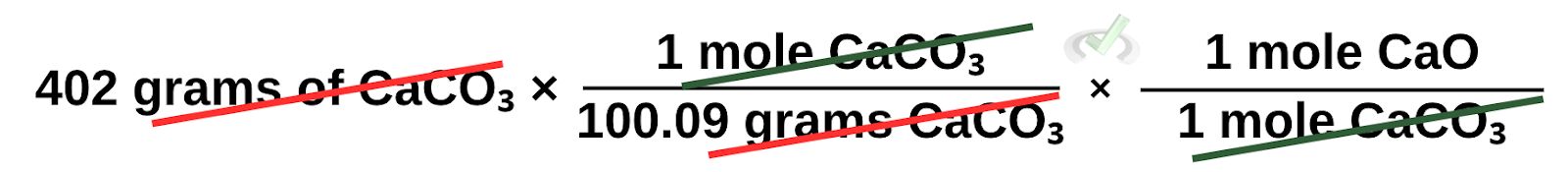
Our unit is now in moles of CaO. However, more calculations are needed because we want to get the grams of CaO as our final answer. We can do this by multiplying it by its molar mass. This time, we do not have to flip the molar mass into its reciprocal since multiplying the moles of CaO by the molar mass in grams per mole cancels moles of CaO.

Our final unit is now in grams. This completes our equation!

The final answer we get is 225.24 grams of CaO produced from 402 grams of CaCO₃. This checks out because we ultimately removed the units we did not want out of using the correct conversion factors.
Let’s try something a little more challenging. Say we have this chemical equation:
Al + HCl → AlCl₂₃ + H₂
And we want to know how many grams of hydrogen gas (H₂) will be produced from 205 grams of aluminum consumed.
First, before starting any calculation, we have to make sure that we have a balanced chemical equation. Evidently, the equation we have is still not balanced since we have 1 hydrogen and chlorine on one side and 2 hydrogen and 3 chlorine atoms on the other. The balanced chemical equation for this problem would be:
2Al + 6HCl → 2AlCl₃ + 3H₂
The problem says we have 205 grams of Aluminum consumed and we want to find the amount of H₂ produced from the reaction. Similar to what we did earlier, we multiply aluminum by the reciprocal of its molar mass.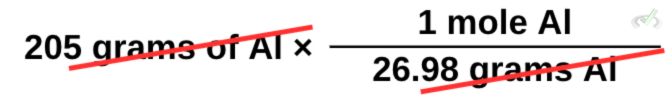
Next, we multiply it by the moles of H₂ that are produced from the moles of Al. From the balanced chemical reaction, we find that 3 moles of H₂ form from 2 moles of Al.

We then multiply this by its molar mass, so we end up with grams of H₂.
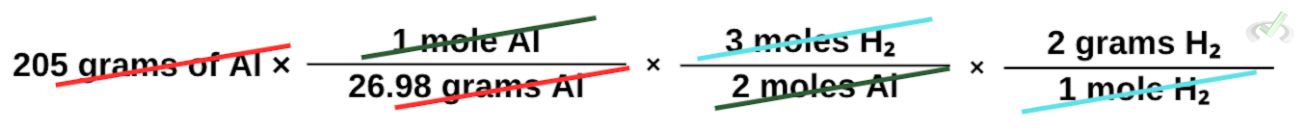
Our final equation would be:

Therefore, 205 grams of Al will make 22.79 grams of H2.
II. Conclusion
In solving calculations in chemical equations, the essential skill we have to master is stoichiometry. Solving chemical equations is merely using appropriate conversion factors to arrive at the unit we want to have. For a typical chemical reaction, we must first ensure that we have a balanced chemical equation at hand. This allows us one more appropriate conversion factor since having a balanced chemical equation gives us the number of moles of reactant that produce a specific number of moles of product. Another conversion factor we can use is the molar mass of a molecule. Writing chemical equations with the appropriate conversion factors simplifies figuring out the value we need.
III. Key Terms
- Conversion factor- A value that changes a unit by multiplying or dividing.
- Stoichiometry- Shows the relationships between the amount of products and reactants in a chemical reaction.
IV. Practice Questions
For items 1-2, use the chemical reaction: CS₂ + O₂ → CO₂ + SO₂
Sample Practice Question 1
If we want to know how many grams of CS₂ are required to make 32 grams of SO₂, what is the first thing we must do?
A. Find the conversion factors appropriate for the equation.
B. Balance the chemical reaction.
C. Use the molar mass of SO₂ as a conversion factor.
D. Determine the specific gravity of SO₂.
Ans. B
Sample Practice Question 2
How many grams of CS2 is required to make 32 grams of SO₂?
A. 10.92 grams
B. 20.19 grams
C. 19.02 grams
D. 21.09 grams
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
