We’ve previously touched on what chemical equations look like. We know that a chemical reaction is a type of reaction that changes the chemical makeup of something. Let’s look at the chemical reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), shown below.
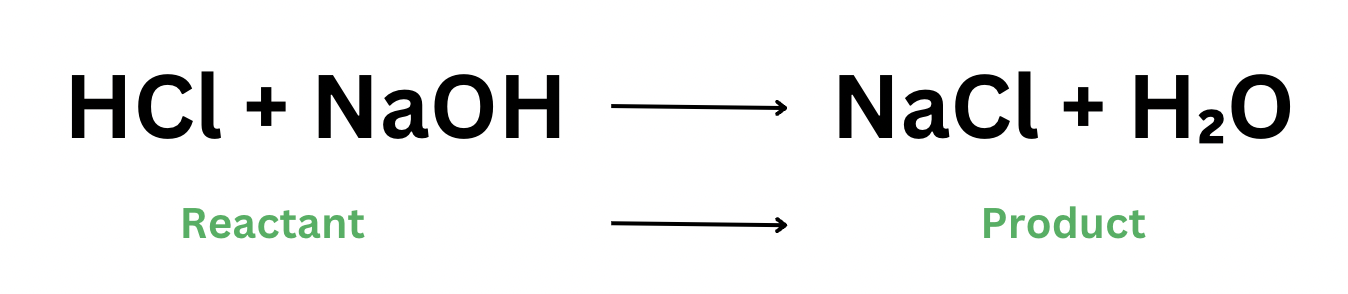
First, we see that we have two reactants: HCl and NaOH. Reactants are the starting point of a chemical reaction. They are the ones that will undergo change. The arrow is a symbol to show how the reactants transform.
In this reaction we see that the reactants completely change into sodium chloride (NaCl) and water (H₂O). In this case, NaOH and H₂O are the products of the reaction as a result of a double-displacement reaction. The reactants will turn into the products, NaOH and H₂O.
You might also notice that in a chemical reaction, we’re merely rearranging the reactants into a new form–we’re not adding anything or removing anything in the process. This is due to the Law of Conservation of Mass.
I. Law of Conservation of Mass
“In a chemical reaction, matter cannot be created nor destroyed.”
This is the fundamental statement of the law of conservation of mass. Looking at it plainly, you’ll notice how it talks about creation and destruction. Chemical reactions talk about chemical change. These changes occur in the participating chemical species. Anything can happen to them–they can bond with other reactants, or remove themselves from a bond, they cannot just disappear or turn into another new thing.
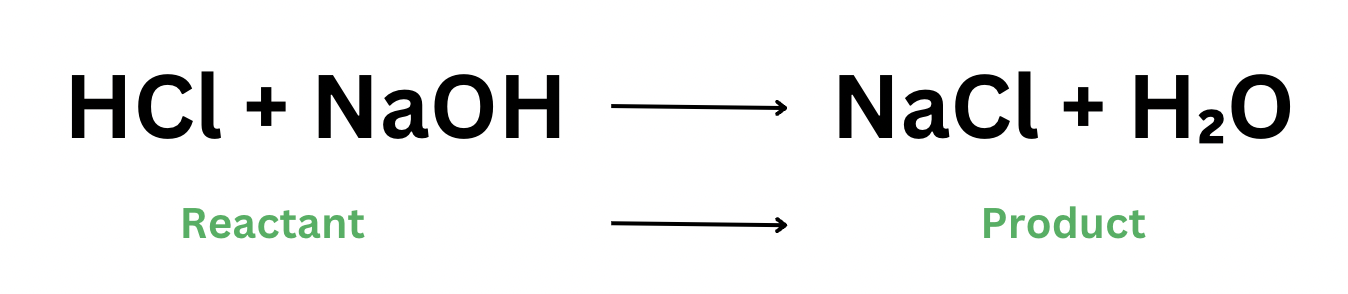
Taking the reaction above as an example, notice how we have four participating elements. We have hydrogen, chlorine, sodium, and oxygen. In this chemical reaction, the reactants merely changed their arrangement. Chlorine from hydrochloride bonds with sodium and the hydrogen and oxygen (OH) moves to bond to the hydrogen from hydrogen chloride. Nothing leaves or enters in this reaction–everything is the same. The chemical species were merely rearranged.
This concept is important because this is the basis for balancing chemical equations. We have to remember that the number of species on the reactants’ side must always be the same number of species on the products’ side. Here, on the reactants’ side, we see 2 hydrogen atoms, 1 chlorine, 1 sodium, and 1 oxygen. The same number of species is also on the products’ side–nothing gets added or removed.
II. Balancing Chemical Equations
Now I know this might be a lot to take in but we can take a different approach to understand how this matters in chemical equations by balancing chemical equations.
The concept of balancing is plain and simple, we balance the number of reactants and products so that the chemical equation we end up with has the same number of products and the same number of reactants. Let’s take this equation as an example:
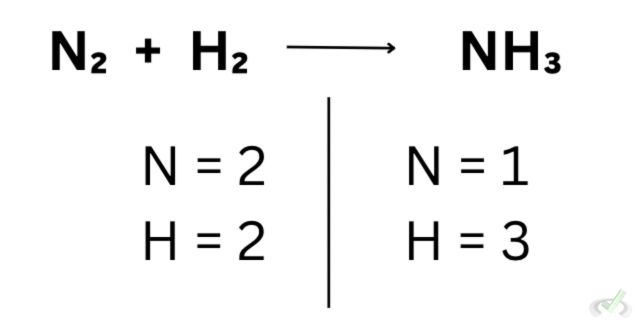
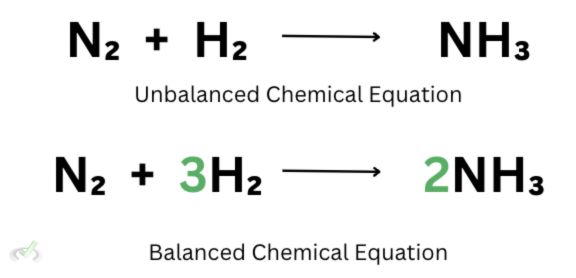
Notice how this equation does not have the same number of atoms on the reactants’ and products’ sides. Since we have 2 nitrogen atoms on one side, let’s add 2 on the other side to make it even.
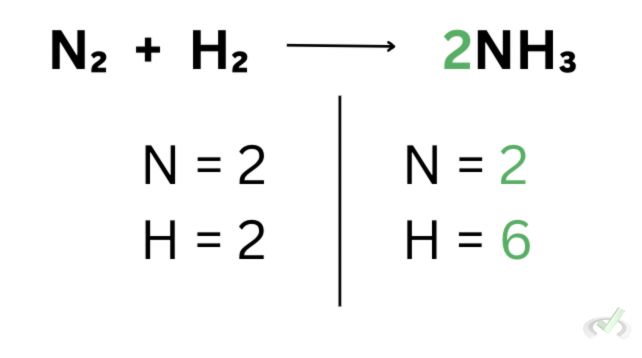
Now that we’ve done that, the number of atoms on the products’ side changes. We now have 2 nitrogen atoms and 6 hydrogen atoms. The six hydrogen atoms come from being multiplied by 2. Since we already had three hydrogen atoms, adding 2 makes this value twice as much.
We also have another problem because while the nitrogen atoms are equal, the hydrogen atoms on one side are still not equal to 6. To remedy this, we can put 3 on the hydrogen to multiply 3 by 2 and have 6 hydrogen atoms on the reactants’ side.
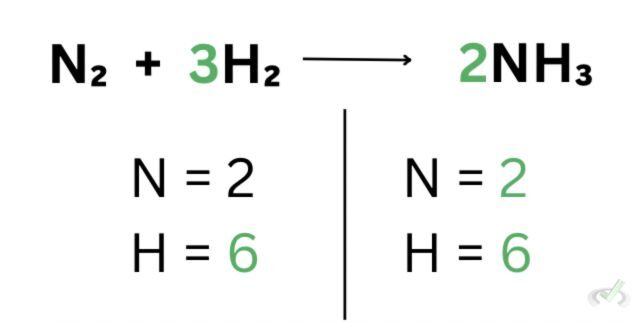
This time, we have 2 nitrogen and 6 hydrogen atoms on both sides. We can now say that we have a balanced chemical equation because the number of reactants is the same as the number of products.
As mentioned in the law of conservation of mass, matter cannot be created nor destroyed. The chemical equation we began with is unbalanced since we had 1 less nitrogen and 3 hydrogen atoms on the products’ side. If we left the equation at that, that would mean that 1 mole of nitrogen disappeared and one hydrogen atom suddenly popped up.
In the law of conservation of mass, we have to account for every single participating chemical species since nothing could have just disappeared or popped up in the process.
III. Other Examples
Let’s try a few examples.
1. C₃H₈ + O₂ → CO₂ + H₂0
Again, we still have an unequal number of species on both sides. We can begin with putting 3 to have an equal number of carbon atoms on both sides. Because of this, we make the number of oxygen 6. We still have 8 hydrogen atoms on one side so we can try to balance this by putting 4 in front of water.
C₃H₈ + O₂ → 3CO₂ + 4H₂0
This makes 8 hydrogen atoms and 3 carbon atoms on both sides. This bit is already balanced. However, we still have to balance oxygen. On the reactants’ side, we have 2 oxygen atoms and on the other, we have 10 oxygen atoms. Since we only have to make these equal, we can put 5 in front of oxygen to have 10 atoms on both sides.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂0
Now, we have a balanced chemical equation. We have 3 carbon atoms, 8 hydrogen atoms, and 10 oxygen atoms on both sides.
2. P + O₂ → P₂0₅
For this, we can begin by putting 2 in front of phosphorus. This makes the number of phosphorus equal on both sides.
2P + O₂ → P₂0₅
However, we still have 2 oxygen atoms on one side and 5 on the other. We can try to add 2 in front of the product so we can change the oxygen on the other side.
2P + O₂ → 2P₂0₅
Now, we have 10 oxygen atoms on the products’ side. We can rectify this by putting 5 in front of the oxygen on the reactants’ side to have 10 oxygen atoms on both sides. Since the number of phosphorus atoms is now unequal, we can change the number in the reactant to 4 to have an equal number of atoms.
4P + 5O₂ → 2P₂0₅
This is our balanced equation. We have 4 phosphorus and 10 oxygen atoms on both sides.
3. FeCl₃ + NaOH → Fe(OH)₃ + NaCl
We can start to balance this equation by making the chlorine atoms equal for both sides. We do this by putting three in front of NaCl so the number of chlorine atoms on both sides is equal.
FeCl₃ + NaOH → Fe(OH)₃ + 3NaCl
By doing this, we also make 3 sodium (Na) atoms on the products’ side. To balance this, we can also do the same to sodium on the other side by putting 3 in front of NaOH.
FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaCl
This neatly balances our equation. We now have 1 iron, 3 chlorine, 3 sodium, 3 oxygen, and 3 hydrogen atoms.
IV. Conclusion
The law of conservation of mass is a fundamental principle in chemical reactions. It basically states that in any chemical reaction, no chemical species can just disappear or a new chemical species can appear. This is important in balancing chemical equations. We have to make sure that whatever is present on the reactants’ side should also be present on the products’ side. This makes balancing chemical equations important. This will contain all of the important ratios and proportions of chemical species in chemical reactions.
V. Key Terms
Law of Conservation of Mass - states that matter is neither created nor destroyed.
V. Practice Questions
Sample Practice Question 1
Balance the chemical equation: CH₄+Cl₂ → CCl₄ + HCl
A. 4CH₄+8Cl₂ → 4CCl₄ + 4HCl
B. CH₄+4Cl₂ → CCl₄ + 4HCl
C. 2CH₄+Cl₂ → 2CCl₄ + HCl
D. 2CH₄+Cl₂ → 2CCl₄ + 4HCl
Ans. B
Sample Practice Question 2
Which of the following is not a balanced chemical equation?
A. 2Al + 6HCl → 2AlCl₃ + 3H₂
B. 2HgO → 2Hg + O₂
C. S₈ + 12O₂ → 8SO₃
D. 2Zn + 4HCl → 2ZnCl₂ + H₂
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
