We use many terms to define how much stuff there is in something. In chemistry, we consider things on a much smaller scale. We look at atoms and molecules and how they can be weighed using a lot of terms. In this article, we’ll explore the key differences in the terms we use to quantify matter and figure out how each term relates to the other.
I. Atomic Weight
We defined atomic weight as an atom's number of protons and neutrons. If we get into its more specific definition–atomic weight is the average of all the masses of naturally occurring isotopes of an element.
This means that when we look at the atomic weight of an element in the periodic table, we’re looking at the average of all of the atomic masses of all its isotopes. Remember that isotopes are forms of an element with a different atomic mass. Isotopes differ in the number of neutrons. Since protons are unique to an element, the number of neutrons makes each isotope unique.
Isotope | Atomic mass | % Abundance | Atomic Mass x % Abundance |
|---|---|---|---|
Carbon-12 | 12 | 98.89 | 11.8668 |
Carbon-13 | 13 | 1.11 | 0.1443 |
Carbon-14 | 14 | <0.00001 | 0.00012 |
Sum/Atomic Weight | 12.0112 | ||
Let’s use carbon as an example. Carbon-12 is 98.89% abundant in nature, Carbon-13 is 1.11% abundant, and Carbon-12 is the least abundant of the three isotopes, with an abundance of less than 0.00001%. Each of these isotopes is noted alongside its atomic mass.
It’s important to note that atomic mass and atomic weight mean different things. Atomic weight is the average atomic mass of all the naturally occurring isotopes. Atomic mass, on the other hand, defines the isotopic mass. This is why carbon-12 has an atomic mass of 12 as opposed to carbon-13 and carbon-14, which have 13 and 14 as their atomic masses, respectively.

When we get the sum of their atomic masses multiplied by their abundance, we get the atomic weight of carbon, which is 12.011 atomic mass units (amu). We use atomic mass units since we cannot use a unit such as grams to define a small quantity. It’s also much more straightforward to visualize since we take 1 unit as 1 proton or neutron. We no longer consider electrons because they’re tiny and do not contribute much to an element’s weight.
II. Molecular Weight
Molecular weight is just how it sounds: It’s the weight of a molecule! A molecule is simply the thing that forms after chemical bonding. When atoms bond with each other, they create a molecule!
Water
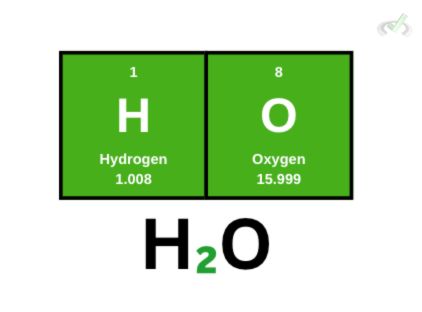
Let’s take a water molecule (H₂O) as an example. Here, we have 2 hydrogen atoms and one oxygen atom. To get its molecular weight, we must get the sum of all the individual atoms in the molecule.
Since we have 2 hydrogen atoms, we take the weight of each atom and add it to the weight of 1 oxygen atom.
(2 x 1.008)+(1 x 15.999) = 18.006 amu
This means that the molecular weight of water is 18.006 amu.
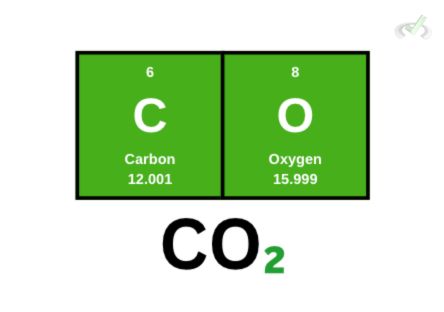
Carbon Dioxide
This time, let’s try carbon dioxide. We have 1 carbon atom and 2 oxygen atoms. To get its molecular weight,
(1 x 12.001)+(2 x 15.999) = 43.999 amu
We can round up the atomic weight of oxygen and round down carbon for simplicity. If we do this, we get:
(1 x 12)+(2 x 16) = 44 amu
Both molecular weights are fine and would still mean the same thing.
Glucose (C₆H₁₂O₆)
Let’s use a more complicated molecule, glucose. Glucose has 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. When we calculate its molecular weight, we get the following:
(6 x 12)+(12 x 1)+(6 x 16) = 180 amu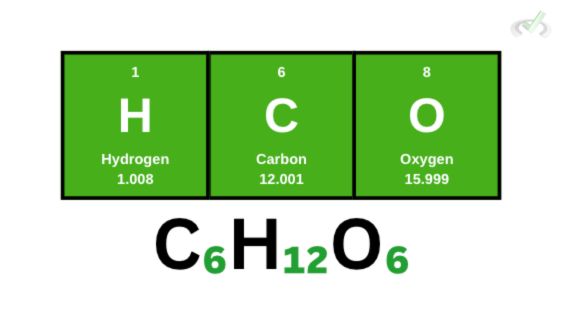
III. Mole
A mole is equal to 6.022 x 10²³ things.
Yes, you heard it right—things. Things can be anything–atoms, molecules, or ions. We use moles to define how much stuff we have.
Let’s use this chemical equation:
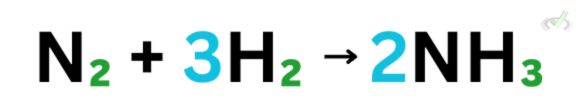
When we try to define how many reactants we have directly, we usually think that we have 2 nitrogen atoms and 3 times 2 hydrogen atoms to yield 2 ammonia (NH3) with 1 nitrogen and 3 oxygen each. Now, that’s just a handful of words to remember, and it does not make sense to still take note of all of the individual atoms, multiply them, and do the same thing for the product.
A mole is a placeholder to simplify quantities that we cannot really define. Instead of saying the whole bit, we can simply say that on the reactants’ side, we have 1 mole of N2 and 3 moles of H2 and on the product’s side, we get two moles of NH3.
Now, why do scientists think this works? Well, on the complicated side of things, when we define the specifics of how much stuff there is, we have to consider each element's weight and multiply it by how many elements there are. If we want to describe something as simple as how much stuff we need, we do not have to describe it by the stuff that makes it up. We can simply say we have 1 mole of this react with 3 moles of this other thing to make 2 moles of that.
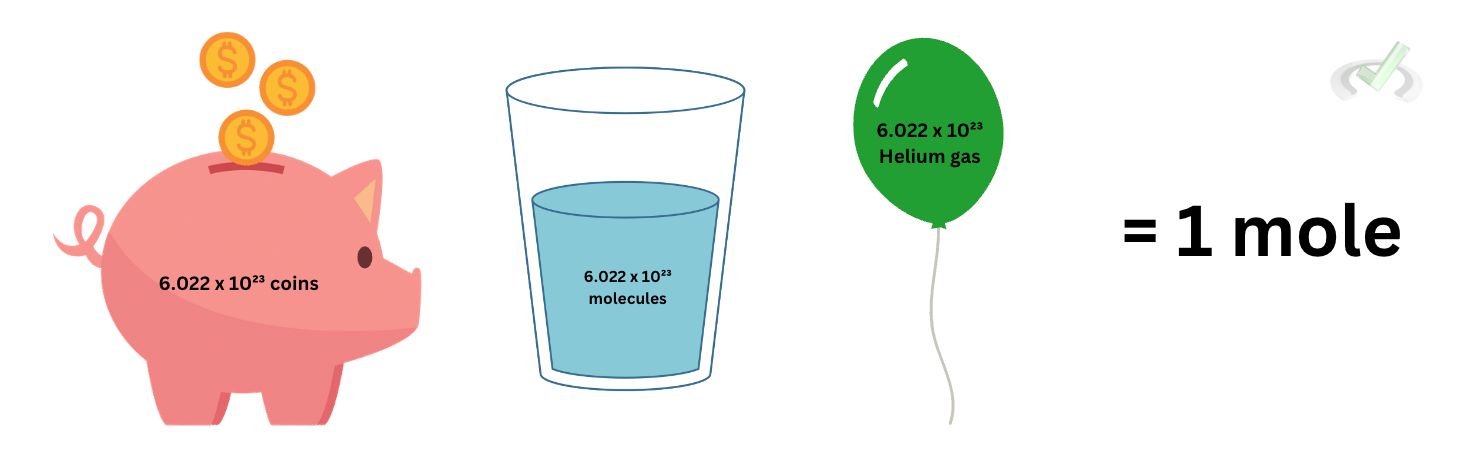
Looking back at how we defined the mole before, we likened it to saying a dozen eggs instead of 12 eggs. This is the concept of the mole. If we want to know how many water molecules are there in one cup of water, there is no way we can look at that water, weigh it, and just say that since we have 250 grams of water, we have 250 molecules of water because, in reality, molecules are tiny. To add to that, atoms are much smaller!
For scientists and us who are just interested in relating quantities that we need, we do not really need to know how much of something we have relative to its weight or ratio. A mole is just a way to define how much stuff is there. The value 6.022 x 10²³ things is equal to one mole. We do not have to focus on this number; all we need is to know that 1 mole means a unit of a number so big that we cannot really be bothered by writing an exponent for it.IV. Molar Mass
Molar mass is the mass in grams of 1 mole of a substance. Nope, we do not have to multiply6.022 1023 to it, we ignore this value and just think that it’s the mass per mole of a substance. We already know that there are 6.022 1023 things in a mole–there is no need to think about it now.
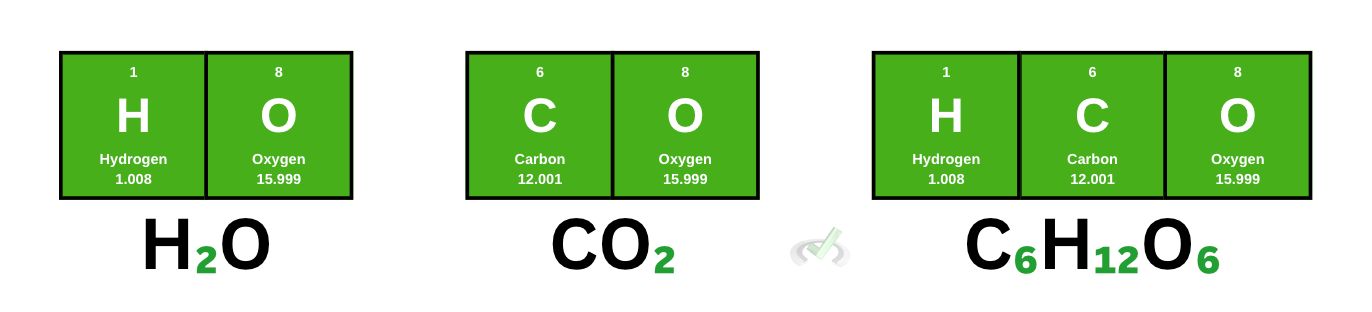
Molar mass is the same as molar weight. The only difference is this time, we use grams per mole (g/mol) instead of amu as our unit. The molar mass of water is 18 g/mol, that of carbon dioxide is 44 g/mol and for glucose, it's 180 g/mol.
Let’s look back at the chemical reaction we discussed earlier and use what we know.
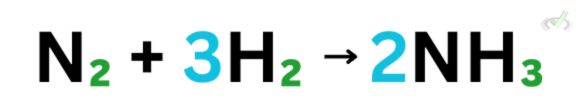
Here, we need 1 mole of N2 and 3 moles of H2 to make 2 moles of NH3.
If we want to get the weight of each atom, we can do this by multiplying the number of moles by its molar mass so we are left with the weight in grams.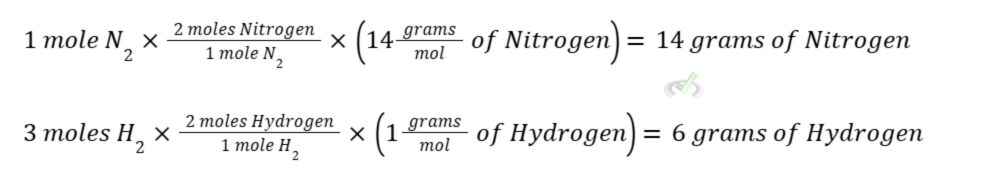
To get the weight of ammonia, we simply multiply the molar mass of ammonia by how many moles of ammonia are present. To get the molecular weight of ammonia, we simply add the atomic weight of one nitrogen and three hydrogen atoms (14+3 = 17 g/mol).
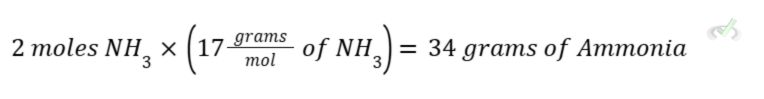
V. Conclusion
We use numbers to quantify matter. This is particularly important in chemistry, especially in the laboratory. Atomic weight is simply the number we see on the periodic table; it’s calculated by considering the average of the atomic masses of all of the naturally occurring isotopes of an element. Molecular weight is the sum of the individual atomic weights in a molecule. Moles, however, are quantities that represent how much stuff there is. We use moles to simplify how we define large quantities. Molar mass, on the other hand, is simply the molecular weight in grams per mole of a molecule.
VI. Key Terms
- Atomic Mass - The mass of an isotope.
- Atomic Weight - The average of the masses of naturally occurring isotopes of an element.
- Molar Mass - The mass of a molecule in atomic mass units.
- Mole - A unit used to define how much stuff is present. A mole is numerically equivalent to 6.022 1023 molecules, atoms, ions, etc.
- Molecular Weight -The molar mass of a molecule in grams per mole.
VII. Practice Questions
Sample Practice Question 1
Which of the following is incorrect?
A. The molecular weight of water is 18 g/mol.
B. The atomic mass is the isotopic mass.
C. The atomic weight considers the abundance of an isotope.
D. A mole describes 6.022 1023 units of something.
Ans. A
Sample Practice Question 2
All of the following statements are correct except:
A. 1 mole of water has 2 moles of hydrogen and 1 mole of oxygen.
B. The atomic weight of oxygen is around 16 amu.
C. 1 mole of N2 will react with 1 mole of H2 to make 2 moles of NH3.
D. The molecular weight of NaCl (sodium chloride) is 58.44 amu
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
