Whether we may realize it or not, we actually use things that contain these organic functional groups quite often! We use alcohol to disinfect our tables and drink it when we maybe want to treat ourselves with some nice Napa Valley wine. Though not as obvious as alcohols, we use ethers in the medical field as well most commonly as an antiseptic!
Alcohols are without a doubt one of the most important functional groups not just in organic chemistry but also in biochemistry! Though ethers don’t have the same emphasis for MCAT testing, it’ll still be helpful to know their basics.
This chapter overview will cover basic topics covering alcohols and ethers, both in organic chemistry nomenclature and structure with a slight emphasis on the reactions involving alcohols. Let’s dive in!
Alcohols and Ethers on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
This section can be expected to be tested more compared to other ones due to the emphasis alcohol has on the MCAT. Try and expect around 5-6 questions covering this topic.
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Alcohols and Ethers
When reviewing this section, try and draw the parallels between the content learned here and biochemistry!
As we mentioned above, alcohols are not only a crucial functional group for organic chemistry, but also play a huge role in the context of biochemistry!
1. Basic Structure and Nomenclature of Alcohols and Ethers
The defining characteristic of an alcohol is the presence of a hydroxyl group (-OH) attached to a carbon backbone via a single bond. In addition, due to the hydroxyl group, alcohols are very polar and can participate in hydrogen bonding. However, note that an increasing number of hydrocarbons will decrease their polarity.
When naming alcohols, determine first whether or not the alcohol is the highest priority functional group. If it is, number the carbon atoms to where the hydroxyl group is on the lowest numbered carbon.
Name alcohol accordingly based on the number of carbons using the names for their corresponding alkanes and replacing the “-ane” suffix with “-ol”. Additionally, put the number before the name to indicate the carbon number. Look at the example below!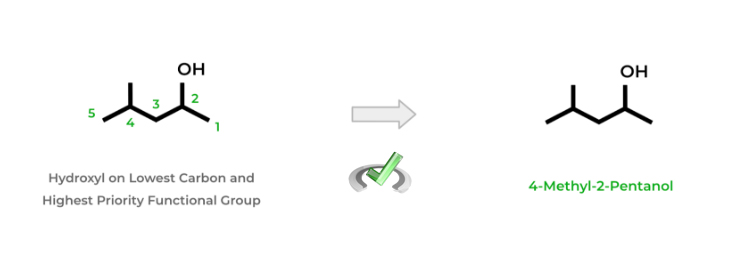
If the hydroxyl group is NOT the highest priority functional group, it’ll just be considered an additional substituent. Identify the hydroxyl groups and which carbons they’re located on. Instead of using the suffix “-ol”, you’ll use the prefix “-hydroxy” because it's of lower priority.
Just like above, put the numbers indicating the carbon location of the hydroxyl groups. Additionally, use the prefixes “-di”, “-tri”, and so on if there’s more than 1 hydroxyl group. Remember to alphabetize when naming!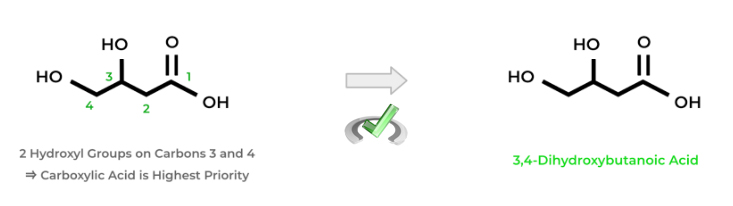
Ethers have a general structure of R-O-R’, where R and R’ refer to the rest of the carbon backbone. It’s much easier to name ethers because they are always considered as the lowest priority substituent, even taking lower priority than alkanes.
To name ethers, identify the functional group on the parent chain. Because the parent chain must be the longest number of carbons, the “R” with the shorter alkyl chain will become what’s called the alkoxy substituent.
Name the alkoxy substituent accordingly based on the number of carbons using the corresponding alkyl substituents. Finally, replace the “-yl” suffix with “-othxy”.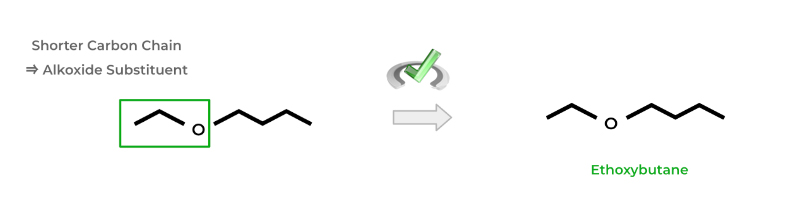
Full Study Notes : Common characteristics, structure, and nomenclature of alcohols and ethers
For more in-depth content review on common characteristics, structure, and nomenclature of alcohols and ethers, check out these detailed lesson notes created by top MCAT scorers.
2. Redox Reactions of Alcohols
Before getting into the general redox reactions of alcohols, let’s quickly review the basics of redox reactions in the context of organic chemistry, which is much more general and simpler compared to general chemistry!
Recall that oxidation in organic chemistry generally means that we’re increasing the number of bonds from a carbon towards oxygen (or a more electronegative atom). Additionally, it also means reducing the number of bonds towards hydrogen.
Conversely, reduction involves increasing the number of bonds from carbon towards hydrogen and decreasing the number of bonds to oxygen (or a more electronegative atom).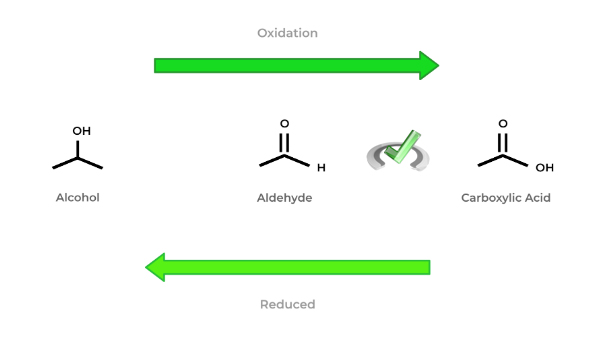
The general rules of oxidation and reduction in organic chemistry are hopefully better seen in the figure above! As we move from an alcohol all the way to the carboxylic acid, the molecule becomes more oxidized.
We can interconvert between these oxidized and reduced states utilizing various oxidizing and reducing agents. Below is a great overview and summation of various oxidizing and reducing agents and the redox reactions they participate in.
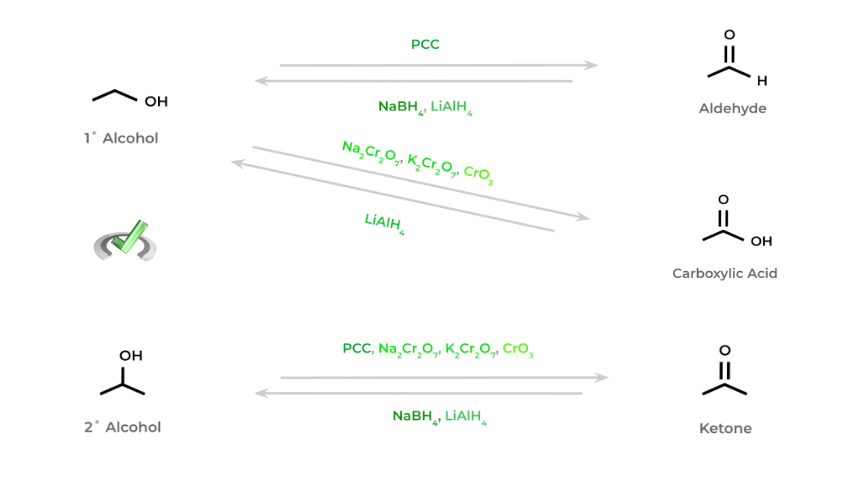
Again, try and notice various commonalities regarding the various oxidizing and reducing reagents. For instance, notice how all the reducing reagents have hydrogen atoms attached! This allows for the reduction of molecules by increasing their bonds to hydrogen atoms!
Full Study Notes : Specifics on the redox reactions of alcohols
For more in-depth content review on specifics on the redox reactions of alcohols, check out these detailed lesson notes created by top MCAT scorers.
3. Protection Reaction of Alcohols
Review for this section is best paired when studying the reactions of aldehydes and ketones, most specifically acetal and ketal formation! We go over a lot more of the specifics in our specialized article and chapter overview for aldehydes and ketones, but we’ll cover just the basics for all you need to know.
The main idea with protection reaction is that diols (alcohols with 2 hydroxyl groups) can react with aldehydes and ketones to generate acetals and ketals. A key characteristic of these functional groups is that they’re generally unreactive.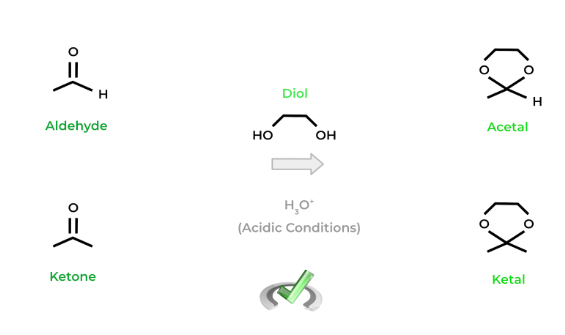
Sometimes in organic chemical reactions, when there are 2 or more functional groups on a molecule, we only want to react to a specific functional group. Look at the molecule below!
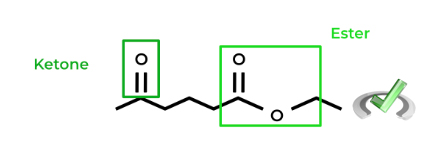
As shown, the molecule above contains a ketone and ester functional group. If we want to reduce ONLY the ester group to an alcohol but not the ketone, we can utilize a diol as a protecting group as we did above!
This is beneficial for a lot of reasons. First, the diol selectively targets the ketone to form a ketal and a protecting group. In addition, because acetals and ketals are generally not reactive, only the ester will be produced. Finally, after reducing the ester, we can remove the protecting group by adding water in acidic conditions.
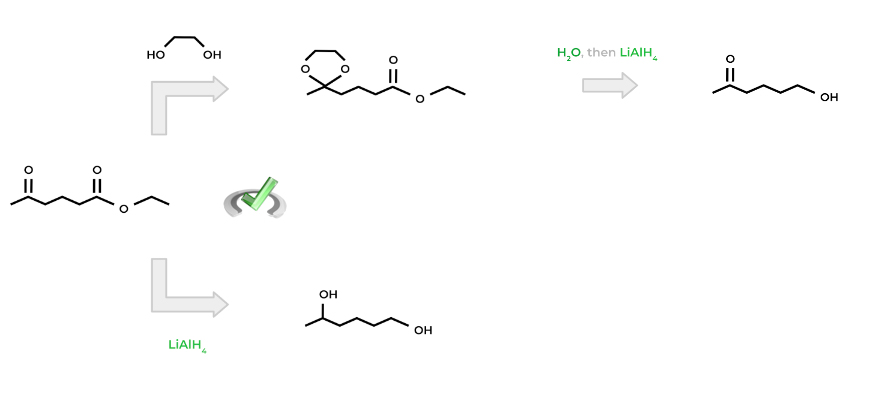
Hopefully, now the term “protecting group” makes a little more sense! Think of it as a “disguise” which hides from the chemical reaction and can be taken off when the reaction is complete!
Full Study Notes : Protection Reaction of Alcohols
For more in-depth content review on protection reaction of alcohols, check out these detailed lesson notes created by top MCAT scorers.
4. Redox Reactions of Phenols
Recall that phenols are basically benzenes (and their derivatives) with a hydroxyl group attached to them. Hence, it combines the 2 terms: “-phen” referring to the phenyl group and “-ol” referring to the alcohol.
Luckily, you won’t have a lot of content to learn or even memorize for this section! This section is more so for your additional understanding and application to metabolic biochemical topics, as you’ll see soon!
Just like other alcohols, phenols can also participate in redox reactions! Look below at the example: the phenol is oxidized utilizing a dichromate salt to its ketone counterpart, called a quinone – specifically, benzoquinone.
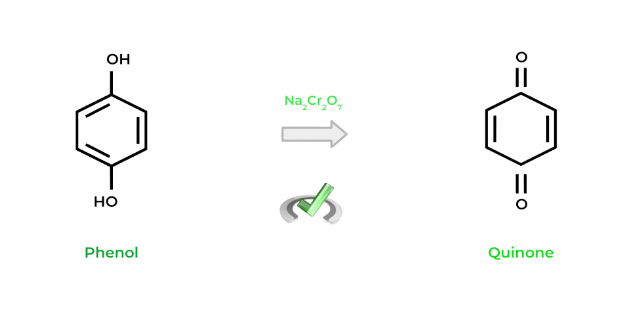
Does the term “quinone” sound familiar by chance? If you’ve covered the oxidative phosphorylation and the electron transport chain, maybe that’s why! Ubiquinol and ubiquinone are examples of phenols and quinones, respectively. Look at their structures below!
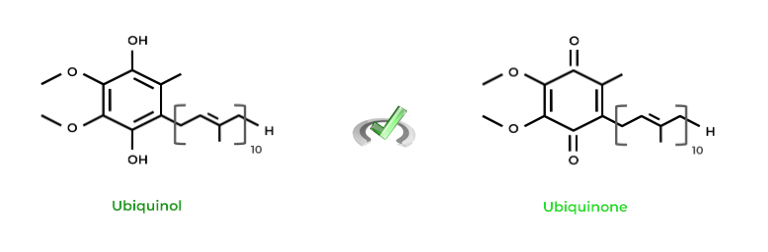
Full Study Notes : Redox Reactions of Phenols
For more in-depth content review on redox reactions that phenols participate in, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about alcohols and ethers in organic chemistry!
Term | Definition |
|---|---|
Alcohol | An organic molecule characterized by the presence of a hydroxyl group bonded to a carbon backbone via a single bond |
Ether | A functional group characterized by the structure R-O-R’, where the 2 R’s refer to carbon backbones |
PCC | Stands for pyridinium chlorochromate (PCC) and is generally characterized as a weak oxidizing agent |
Na2Cr2O7, K2Cr2O7 | Examples of dichromate salts which are generally characterized as strong oxidizing agents |
CrO3 | Also known as the Jones reagent; Generally characterized as a strong oxidizing agent |
LiAlH4, NaBH4 | Stands for lithium aluminum hydride and sodium borohydride, respectively; Generally characterized as strong reducing agents |
Protecting Group | Functional groups, usually cyclic acetals and ketals, which can be used to protect aldehydes and ketones from reactions |
Phenols | Refers to benzene (and its derivatives) molecule with a hydroxyl group attached |
Quinones | Refers to the oxidized, ketone counterpart of phenols |
Additional FAQs - Alcohols and Ethers on the MCAT
What are Alcohols? – MCAT
What are Mesylates and Tosylates? – MCAT
Why Are Tertiary Alcohols Not Easily Oxidized? – MCAT
In order to be oxidized and attach an additional bond to oxygen, one of the carbon-carbon bonds must be broken, which is highly unfavorable. Contrast to a carbon-hydrogen bond which is much easier to break in!
What Functional Groups Do You Need to Know for the MCAT?
Do You Need to Know OChem 2 for the MCAT?
Primarily focus on the main nucleophilic substitution reactions of alcohols, esters, aldehydes, ketones, and carboxylic acids! Also, try to cover the main reactions for carboxylic acid derivatives as we cover in our other chapter overviews and articles!
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
