Stereoisomers are a critical topic in organic chemistry. They include molecules with the same molecular formula but different three-dimensional arrangements of atoms. Diastereomers are a specific type of stereoisomers. Understanding diastereomers is essential for grasping many organic chemistry concepts, especially concerning physical and chemical properties.
I. Basics of Stereoisomers
A. What are Stereoisomers?
Stereoisomers are molecules with similar molecular formulas and sequences of bonded atoms (constitution). However, they differ in the three-dimensional orientations of their atoms. These differences can significantly impact the molecules' properties and reactivity. For example, they might have different melting, boiling, or solubilities.
B. Types of Stereoisomers
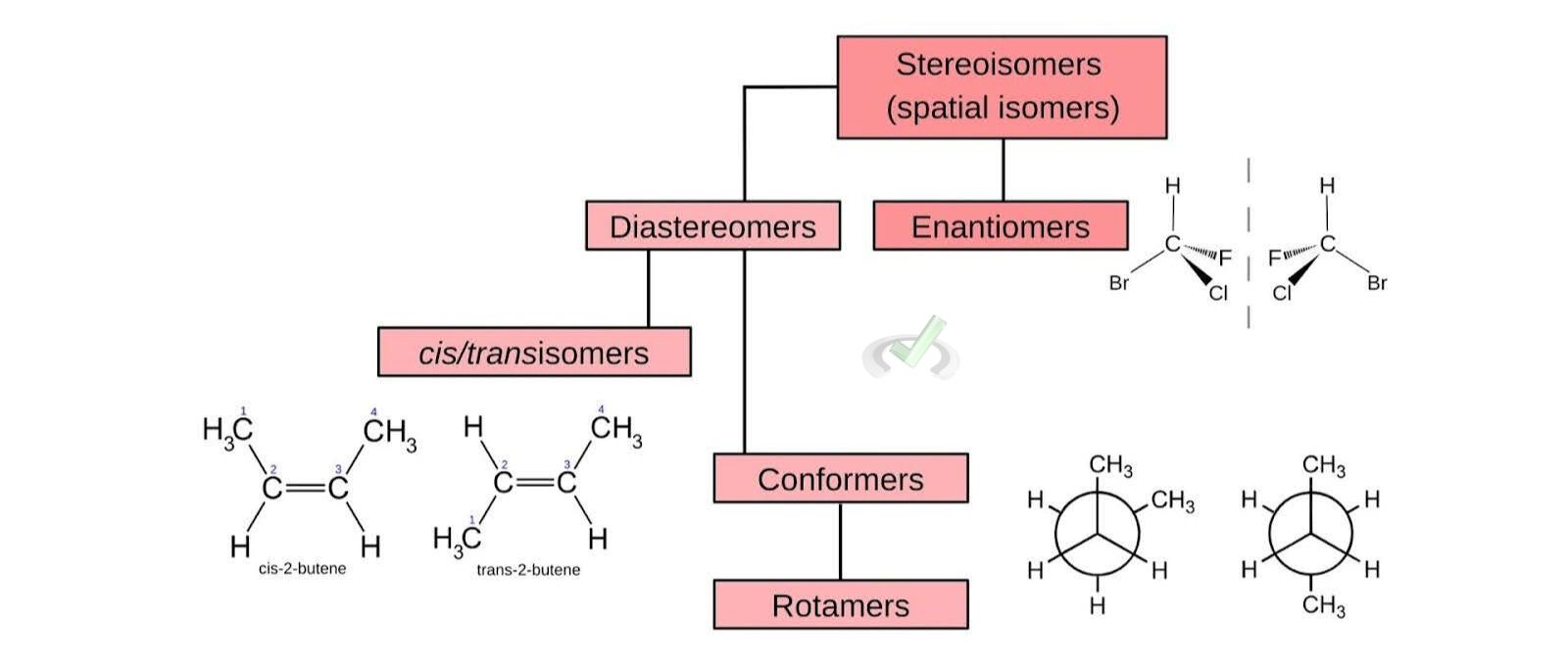
- Enantiomers: These are non-superimposable mirror images of each other. Think of your left and right hands. They are similar but not identical. An example is the pair of D- and L-glucose.
- Diastereomers: These are stereoisomers that are not mirror images of each other. They have different physical properties and reactivity. For instance, cis and trans isomers in alkenes.
- Conformers (Conformational Isomers): These are stereoisomers that differ by rotation around a single bond. They can interconvert by rotating about the bond. For example, the different staggered and eclipsed forms of ethane.
- Rotamers: These are a type of conformational isomer where the rotation occurs around a single bond, leading to different spatial arrangements. For instance, the different spatial arrangements of atoms in butane due to rotation around the C-C bond.
II. Diastereomers: A Closer Look
A. Defining Diastereomers
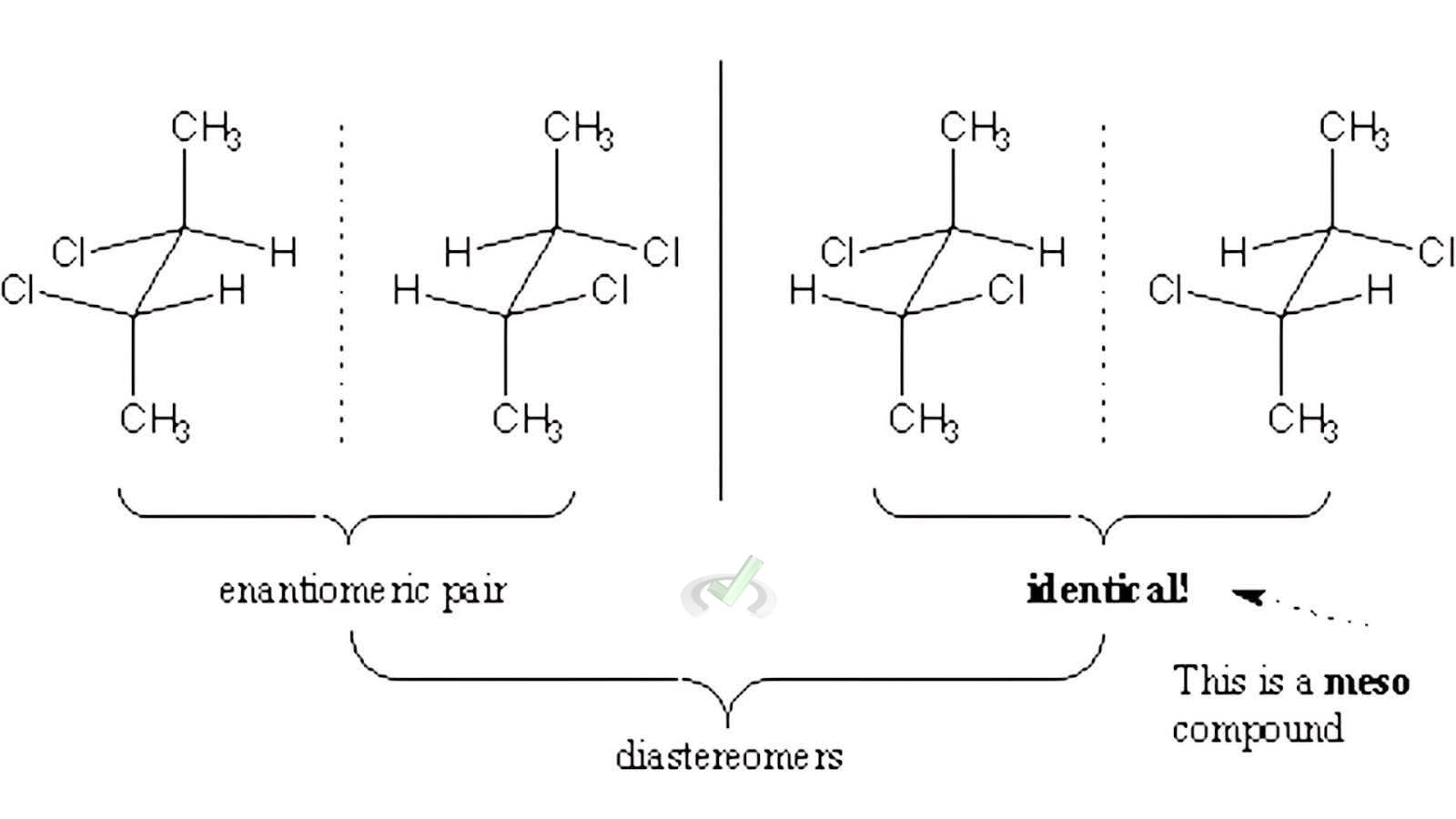
Diastereomers are a type of molecule that has more than one chiral center. A chiral center is like a special point in the molecule that can be arranged differently.
There's also a special kind of molecule called a meso compound. Meso compounds have more than one chiral center, but they are not chiral overall because they have a plane of symmetry. This means you can fold them in half and match them up perfectly, making them superimpossible in their mirror image.
For example, if you have a molecule with two chiral centers and change one while keeping the other the same, you get a diastereomer. But if a molecule with two chiral centers has a plane of symmetry, it is a meso compound and is not chiral even though it has chiral centers.
B. Properties of Diastereomers
- Different Physical Properties: Unlike enantiomers, which are like left and right hands (mirror images), diastereomers are not mirror images of each other. Because of this, diastereomers can have very different properties, like how they look, melt, or react with other substances.
- Chemical Reactivity: Diastereomers often react differently with other compounds, making them useful in various chemical reactions and synthesis processes.
III. How Diastereomers are Formed
A. Formation through Chiral Centers
Diastereomers are formed when a molecule has two or more chiral centers. Each chiral center can exist in one of two configurations (R or S). This leads to multiple stereoisomers, some of which will be diastereomers of each other.
Example: Tartaric Acid (C4H6O6)
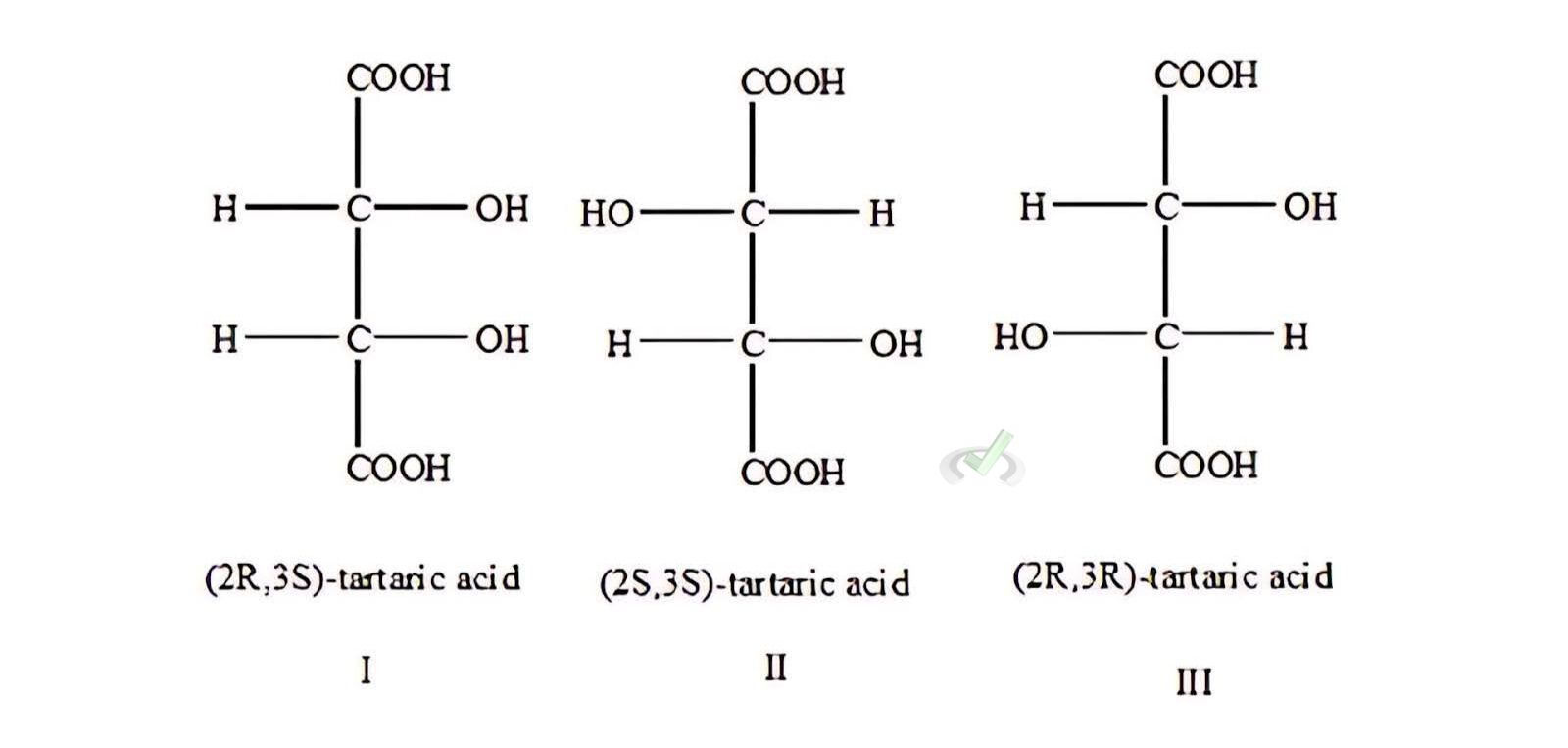
Tartaric acid has two chiral centers. This gives rise to three stereoisomers:
- (R,R)-Tartaric Acid
- (S,S)-Tartaric Acid
- (R,S)-Tartaric Acid
B. Cis-Trans Isomerism
Another type of diastereomerism is cis-trans isomerism, which occurs in alkenes and cyclic compounds. In these cases, the relative positions of substituents on either side of a double bond or ring determine the isomer.
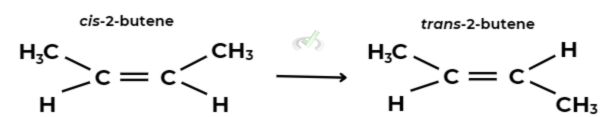
Example: 2-Butene (C4H8)
2-Butene can exist as:
- cis-2-Butene (both methyl groups on the same side)
- trans-2-Butene (methyl groups on opposite sides)
IV. Importance of Diastereomers
A. Biological Relevance
Diastereomers play a crucial role in biological systems. Enzymes and receptors are often chiral. This means they will typically interact with one diastereomer more effectively than the other. This is crucial in drug design and pharmaceuticals.
B. Pharmaceutical Applications
Many drugs are diastereomerically pure or are mixtures where one diastereomer is more active. For example, the drug ephedrine exists in two diastereomeric forms with different levels of activity and side effects.
V. Identifying and Separating Diastereomers
A. Techniques for Identification
- NMR Spectroscopy: This technique can distinguish diastereomers based on their unique chemical environments.
- Chromatography: Due to their different interactions with the stationary phase, diastereomers can often be separated using techniques like high-performance liquid chromatography (HPLC).
B. Techniques for Separation
- Resolution Methods: Diastereomers can be separated using physical methods due to their different solubilities or melting points.
- Chiral Synthesis: Using chiral catalysts or reagents can help produce a specific diastereomer preferentially.
VI. Connecting to Broader Organic Chemistry Concepts
Understanding diastereomers is essential for broader organic chemistry.
A. Formation through Chiral Centers
Diastereomers help in studying reaction mechanisms. Knowing how different isomers form and react helps chemists design more efficient synthetic routes.
B. Stereoselective Reactions
Many reactions are stereoselective, producing one diastereomer preferentially. This is important in synthesizing compounds with desired properties.
C. Stereochemistry in Natural Products
Many natural products are chiral, and their biological activity often depends on their stereochemistry. Understanding diastereomers helps in the study and synthesis of these compounds.
D. Spectroscopy and Identification
Spectroscopy is a technique for identifying compounds. In IR spectroscopy, diastereomers show different absorption peaks due to their unique chemical environments.
NMR spectroscopy can further distinguish between diastereomers based on their chemical shifts and coupling patterns. These analytical techniques are crucial for structure determination.
VII. Wrap-Up and Key Terms
Understanding diastereomers is essential for learning many topics in organic chemistry. They are stereoisomers that are not mirror images of each other. Also, they have different physical and chemical properties.
Key Terms:
- Stereoisomers: Molecules with the same molecular formula but different 3D arrangements.
- Enantiomers: Non-superimposable mirror images.
- Diastereomers: Stereoisomers that are not mirror images.
- Conformers (Conformational Isomers): Stereoisomers that differ by rotation around a single bond.
- Rotamers: Conformational isomers with different spatial arrangements due to rotation around a single bond.
- Chiral Center: An atom, typically carbon, bonded to four different groups.
- Cis-Trans Isomerism: A type of diastereomerism in alkenes and cyclic compounds.
VIII. Practice Questions
Sample Practice Question 1
What is a diastereomer?
A) A molecule that has the same molecular formula as another but a different arrangement of atoms.
B) A non-superimposable mirror image of another molecule.
C) A stereoisomer that is not a mirror image of another molecule.
D) A chiral molecule with one chiral center.
Ans. C
Diastereomers are stereoisomers that are not mirror images of each other. They have different physical properties and reactivity.
Sample Practice Question 2
Which method can be used to separate diastereomers?
A) Distillation
B) Chiral Chromatography
C) Filtration
D) Sublimation
Ans. B
Chiral chromatography can separate diastereomers due to their different interactions with the stationary phase.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
