In this chapter overview, we’re getting subatomic! Though the above title might look scary initially, the underlying concepts are relatively simple to comprehend as we’ll touch on in the sections below!
When going through this chapter overview, you’ll notice that there’ll be a lot of overlap and bridges with the “Atomic Structure” chapter overview located in our General Chemistry module!
In this chapter overview, we’ll cover the basics of atomic structure and the important nuclear reactions that you’ll need to know for the MCAT. Let’s get started!
Atomic and Nuclear Phenomena on the MCAT: What You Need to Know
Topics on atomic and nuclear phenomena will be tested on the Chem/Phys section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try and expect around 3-4 questions about atomic and nuclear phenomena that may appear on the MCAT!
Introductory physics accounts for 25% of the content covered in the Chemical and Physical Foundations of Biological Systems.
Important Sub-Topics: Atomic and Nuclear Phenomena
While we'll go over the basics of atomic structure, this chapter is more focused on the emission spectrum and nuclear reactions that are involved with atoms.
For more specifics on the atomic structure, such as electron configuration, orbitals, etc., check out our “Atomic Structure on the MCAT!” chapter overview!
1. Basics of Atomic Structure
As this is a chapter overview, we’ll keep the content relatively simple! The atom is composed of 3 main subatomic particles: protons, neutrons, and electrons. Protons are positively charged, electrons are negatively charged, and neutrons are neutral (no charge).
The protons and neutrons are located in a dense region in the center of the atom called the nucleus while the electrons are located and constantly orbiting around the nucleus. You might have pictured this structure as the nucleus surrounded by different levels of circles with electrons orbiting!
While certain aspects of this are correct, such as electrons orbiting in different energy levels, current research says that it might be more correct to describe the positions of electrons as an “electron cloud.” Rather than traveling in circular orbitals, electrons don’t have a particular pathway of travel and instead move around randomly within the electron cloud. Try and think of it also like a “fuzz”!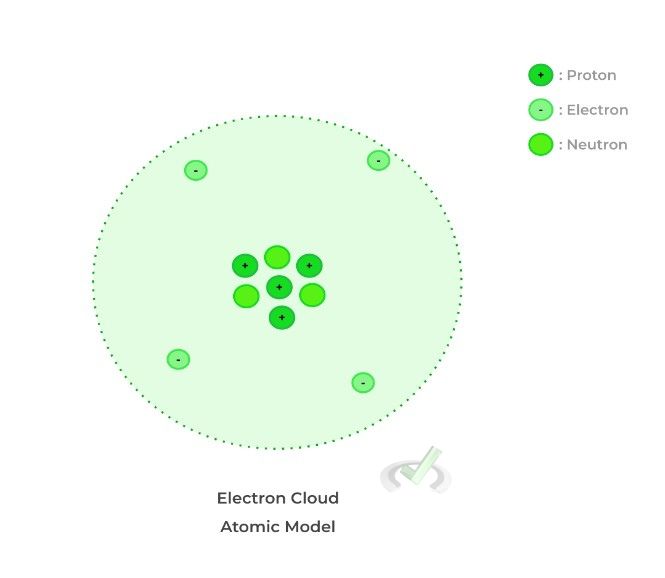
To understand the different types of nuclear reactions and their resulting products, it’s important to differentiate between atomic number and mass number.
The atomic number is simply the number of protons in an atom: this is what determines what element an atom is. The mass number is just the number of protons and neutrons in an atom added together.
Isotopes are a related concept which refers to atoms with the same number of protons but differ in the number of neutrons within the nucleus. In other words, they have the same atomic number but differ in their mass number.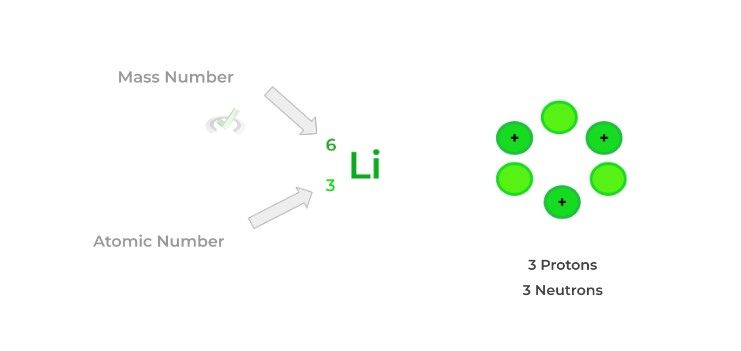
Full Study Notes : Basics of Atomic Structure
For more in-depth content review on the atomic structure in relation to nuclear phenomena, check out these detailed lesson notes created by top MCAT scorers.
2. Atomic Absorption and Emission of Light
An important concept to grasp in atomic structure is that the electron clouds have different energy levels that the electrons occupy, with the level closest to the nucleus being the lowest and increasing as you move outwards from the nucleus.
When discussing the atomic absorption and emission of light, it helps to utilize the simplified hydrogen atom Bohr model as the model depicts different energy levels in a growing circular orbit with one electron. What’s interesting is that the electron can move in between energy levels depending on whether it absorbs or releases energy in the form of photons.
When increasing energy levels, the electron absorbs energy in order to be “excited” and move up energy levels. Conversely, when decreasing energy levels, the electron emits and releases energy!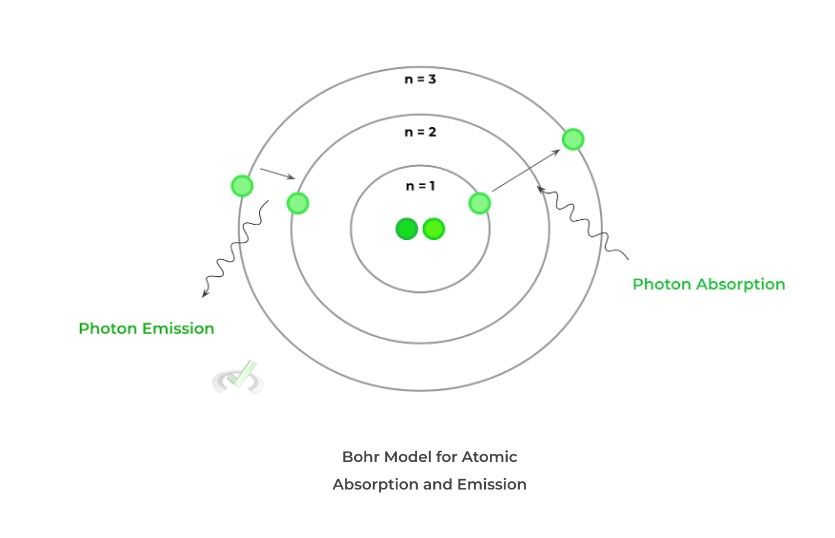
Though we’ll get into many equations and calculations associated with atomic absorption and emission, a high yield one to know is the equation that finds the energy of a photon (emitted or absorbed):
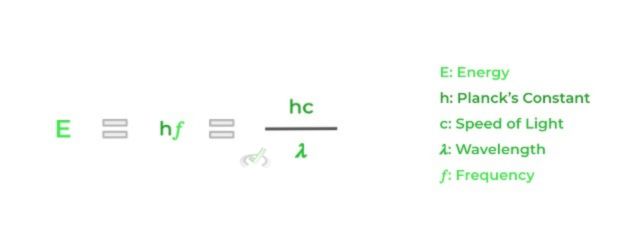
The 2 equations are basically derivatives of one another but both can ultimately be used to find the energy. This is without a doubt a must know equation you want to memorize for the MCAT!
Full Study Notes : Atomic Absorption and Emission of Light
For more in-depth content review on atomic absorption and emission of light, check out these detailed lesson notes created by top MCAT scorers.
3. Nuclear Decay
While there are many types of nuclear reactions, the most common type are nuclear decay reaction. These occur in radioactive isotopes which release particles in order to attain a more stable nuclei configuration. There are 3 basic types of nuclear decay: alpha (𝜶), beta (𝜷) -- divided into positive and negative --, gamma (𝜸).
Only 𝜶 and 𝜷 decays result in a change in the atomic structure of the isotope. An 𝜶- decay results in a release of a helium nucleus with a mass number of 4 (2 protons and 2 neutrons).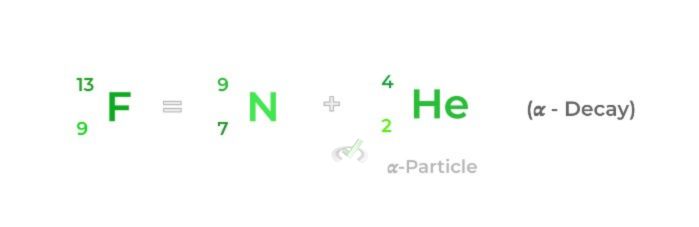
𝜷-decays are a little more complicated as they involve the conversion of a neutron into a proton and vice versa.
A 𝜷+ decay (a.k.a. positron emission) results in a proton being converted into a neutron, decreasing the atomic number by 1. A 𝜷- decay is essentially the opposite, as it results in a neutron being converted into a proton, increasing the atomic number by 1.
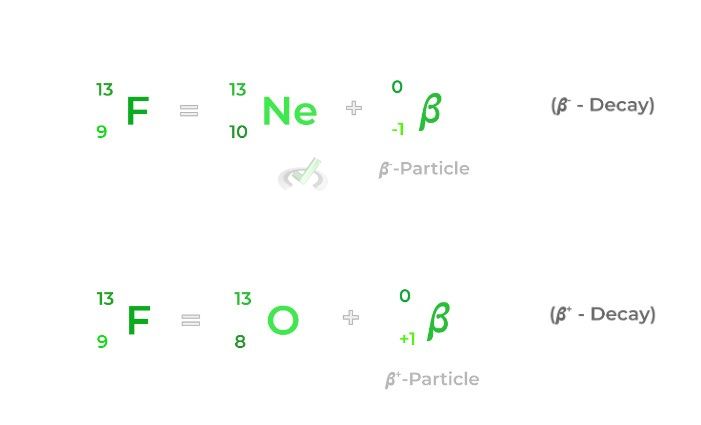
Note that whether a beta decay is positive or negative, the mass number remains the same as the sum of the protons and neutrons remains the same!
𝜸-decay is the only form of radioactive decay that does release a particle from the atom; rather, electromagnetic radiation in the form of a gamma photon is released! Sometimes, an asterisk is included on the parent nucleus to indicate that it’s a high energy molecule.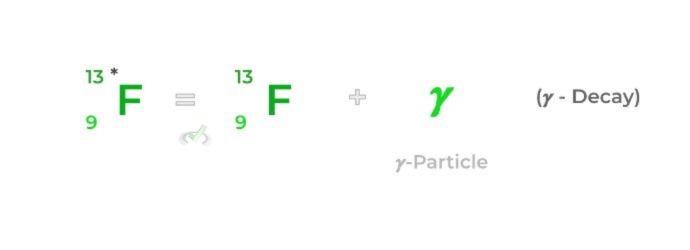
Full Study Notes : Nuclear Decay
For more in-depth content review on nuclear decay, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about atomic and nuclear phenomena!
Term | Definition |
|---|---|
Proton | Positively charged subatomic particle located in the nucleus |
Neutron | Neutrally charged subatomic particle located in the nucleus |
Electron | Negatively charged subatomic particle surrounding the nucleus traveling within the electron cloud |
Atomic Number | The number of protons an atom contains; this determines what element an atom is |
Mass Number | The sum of the protons and neutrons within an atom |
Isotope | Atoms with the same number of protons (same element) but differing in the number of neutrons |
𝜶-Decay | Radioactive decay which results in the emission of a helium atom with a mass number of 4 |
𝜷+- Decay | Radioactive decay which results in the conversion of a proton into a neutron; also called positron emission |
𝜷-- Decay | Radioactive decay which results in the conversion of a neutron into a proton |
𝜸-Decay | Radioactive decay which results in the release of electromagnetic radiation in the form of photons |
Additional FAQs - Atomic and Nuclear Phenomena on the MCAT
Is Nuclear Chemistry on the MCAT?
Is Radioactive Decay on the MCAT?
What is Beta Minus Decay – MCAT?
What is the Difference Between Atomic Physics and Nuclear Physics– MCAT?
Additional Reading Links – Study Notes for Atomic and Nuclear Phenomena on the MCAT
Additional Reading: Physics Topics on the MCAT:
- Circuits on the MCAT
- Electrostatics on the MCAT
- Fluids on the MCAT
- Kinematics on the MCAT
- Light and Optics on the MCAT
- Magnetism on the MCAT
- Thermodynamics on the MCAT
- Units and Dimensional Analysis on the MCAT
- Waves and Sound on the MCAT
- Work and Energy on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
