I. What are Biosignaling Proteins?
While on the surface level we attribute the physiological functioning of our bodies to organ systems and tissues, it’s really at the cellular, biochemical level where all our bodies’ physiological functioning originates and stems from!
We can thank the intricate networks of biosignaling proteins for being the foundation of all our physiological functions! These proteins are responsible for the initiation and regulation of pathways in order to keep our bodies functioning in tip top shape.
Additionally, the beauty of these biosignaling proteins is that wide array of intracellular and intercellular networks that can be made! Just through one google search of cell pathways, you can see and appreciate the complexity put into cellular communications.
II. Different Types of Biosignaling Proteins.
While there are many different types of biosignaling molecules, we’ll focus on two main types: 1) receptors and 2) ion channels.
A. Receptors
The binding of the signaling molecule at the receptor’s ligand binding domain allows for signal transduction which can activate or inhibit a pathway depending on both the signaling molecule and/or the receptor.
Additionally, 2 of the most recognized receptor types are enzyme linked receptors and G protein coupled receptors as we’ll discuss further below!I. Enzyme Linked Receptors
As their name suggests, these receptors actually have dual function as both a receptor and enzyme!
These receptors have 2 special domains: a ligand binding domain to bind the ligand and a catalytic domain, which has enzymatic function!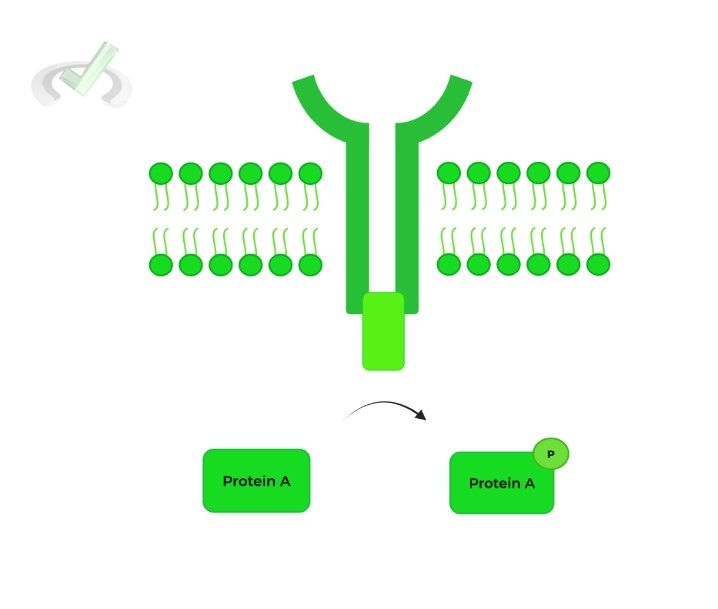
In the example above, the catalytic domain functions as kinase, allowing for the phosphorylation of protein A!
II. G Protein Coupled Receptors
Picture the G protein coupled receptors as having 2 components: actual G protein and the receptor, which connects to the G protein by a protein segment spanning the membrane 7 times.
The G protein is a heterotrimeric protein, meaning it’s composed of 3 different subunits: the alpha (𝛂), beta (𝜷), and gamma (𝞬) subunit.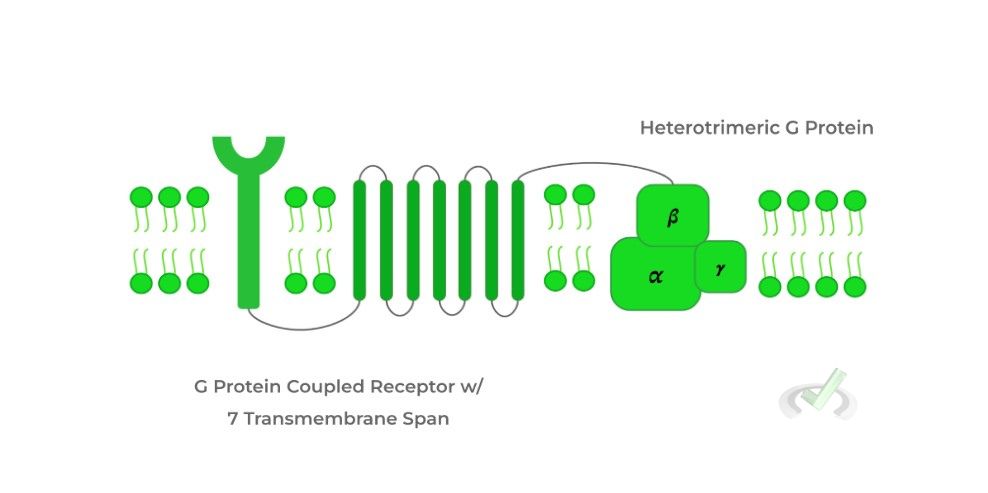
It’s important to note that the alpha (𝛂) in its inactive form is bound to a GDP molecule. Upon binding of the ligand to the receptor, the GDP molecule is swapped for a GTP molecule activating the subunit!
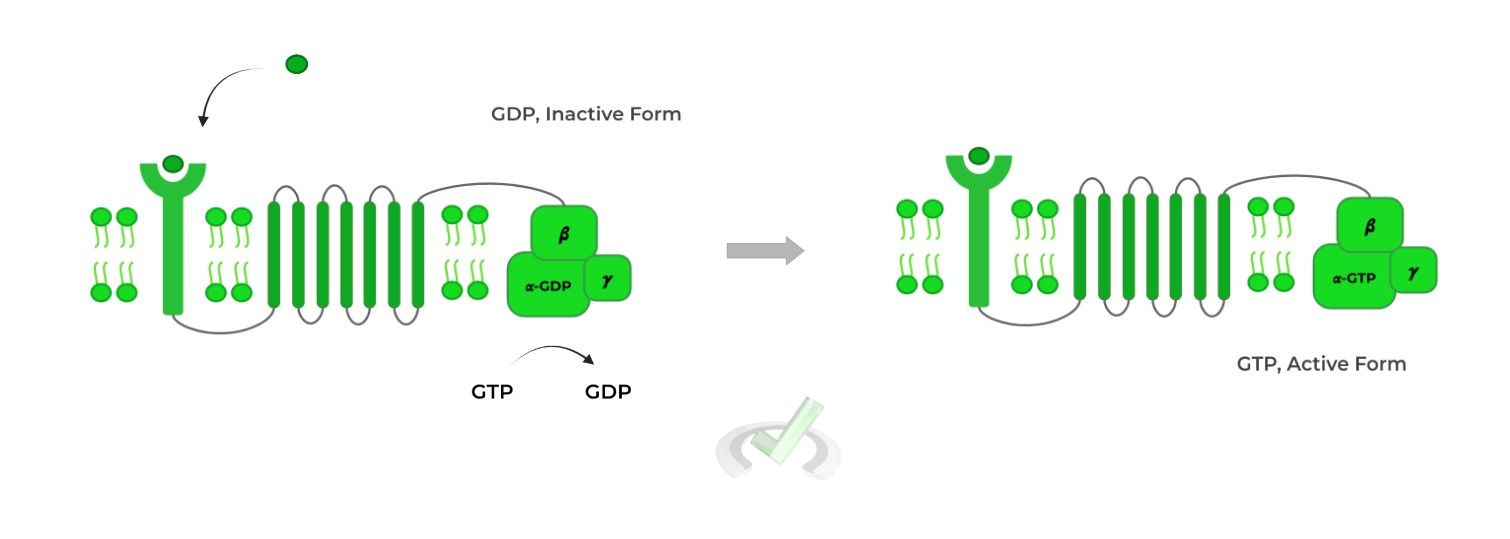
The activated alpha (𝛂) subunit then dissociates from the G protein complex and travels the membrane to activate adenylate cyclase, which converts AMP to cyclic AMP (cAMP).
Cyclic AMP can then go on to activate many other substances, with protein kinase A being a popular target!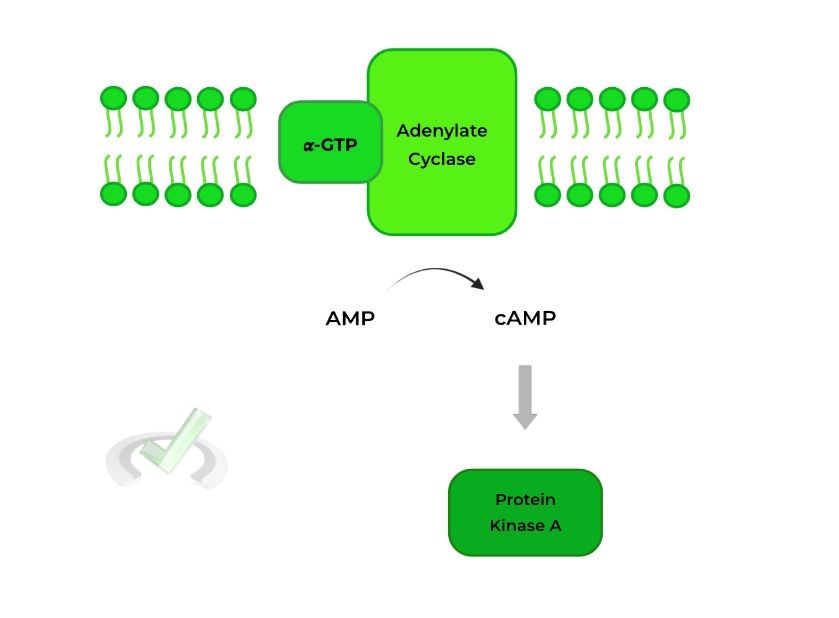
Once the 𝜶-GTP subunit is dephosphorylated, the 𝜶-GDP subunit is reformed and the pathway is terminated.
The outcomes of the pathway can actually differ depending on the alpha (𝜶) subunit. A Gs alpha subunit STIMULATES adenylate cyclase while a Gi alpha subunit INHIBITS adenylate cyclase.B. Ion Channels
The flow of ions mediated by ion channels can also have function in signal transduction! There are 3 main types of ion channels: 1) ungated, 2) ligand gated, and 3) voltage gated ion channels.
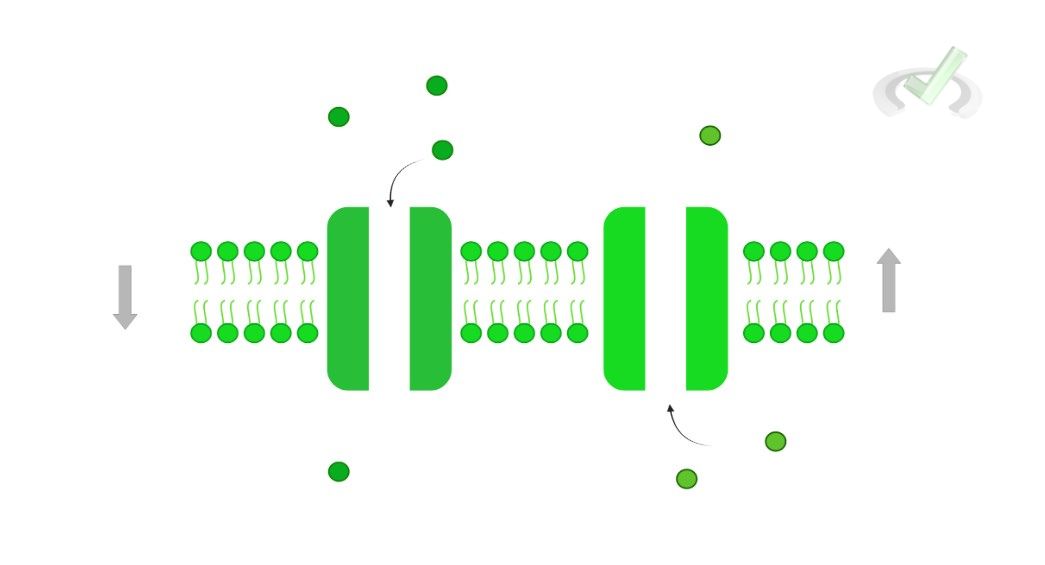
I. Ungated
Also known as leakage channels, ungated ion channels are “always open” allowing for the constant flow of ions down their concentration gradient.
II. Ligand Gated
This can actually also be classified as a receptor! Similar to the G protein, think of this protein as having 2 parts: the receptor and the ion channel.
When the ligand binds to the receptor, a conformational change occurs which opens the ion channel, allowing for the flow of ions!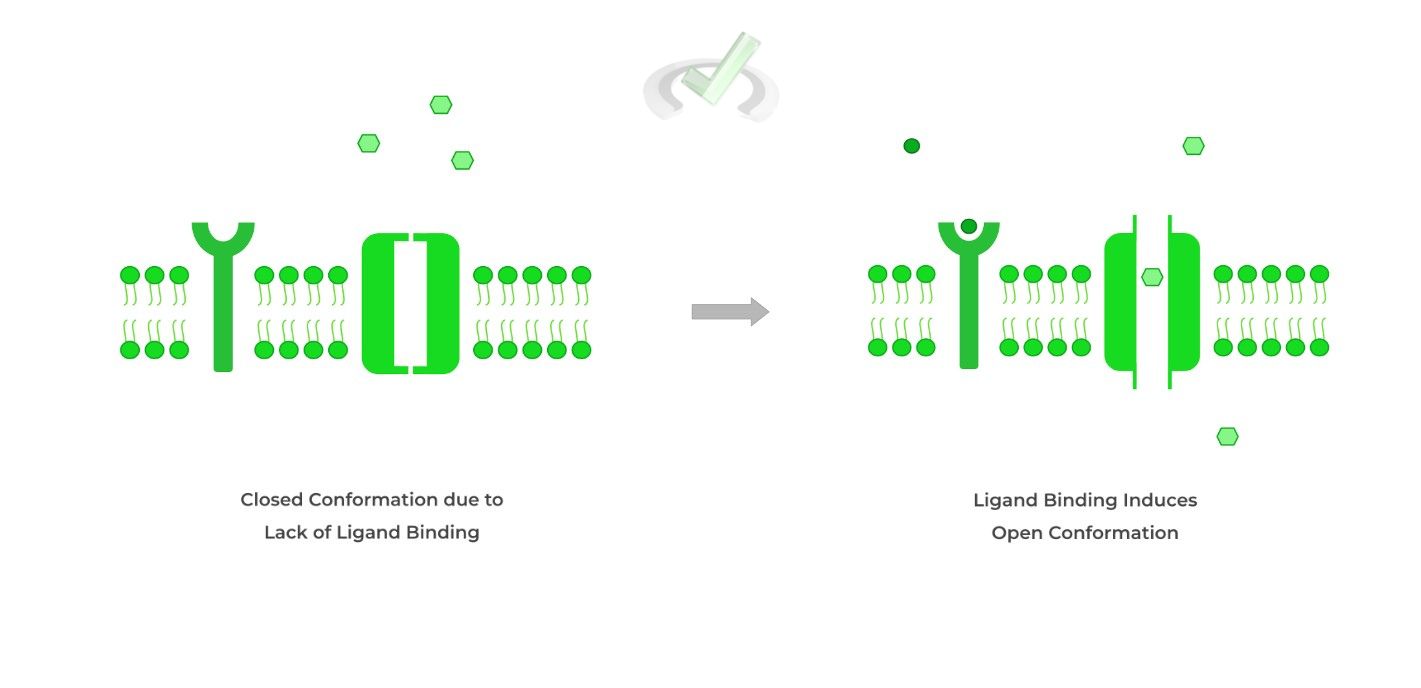
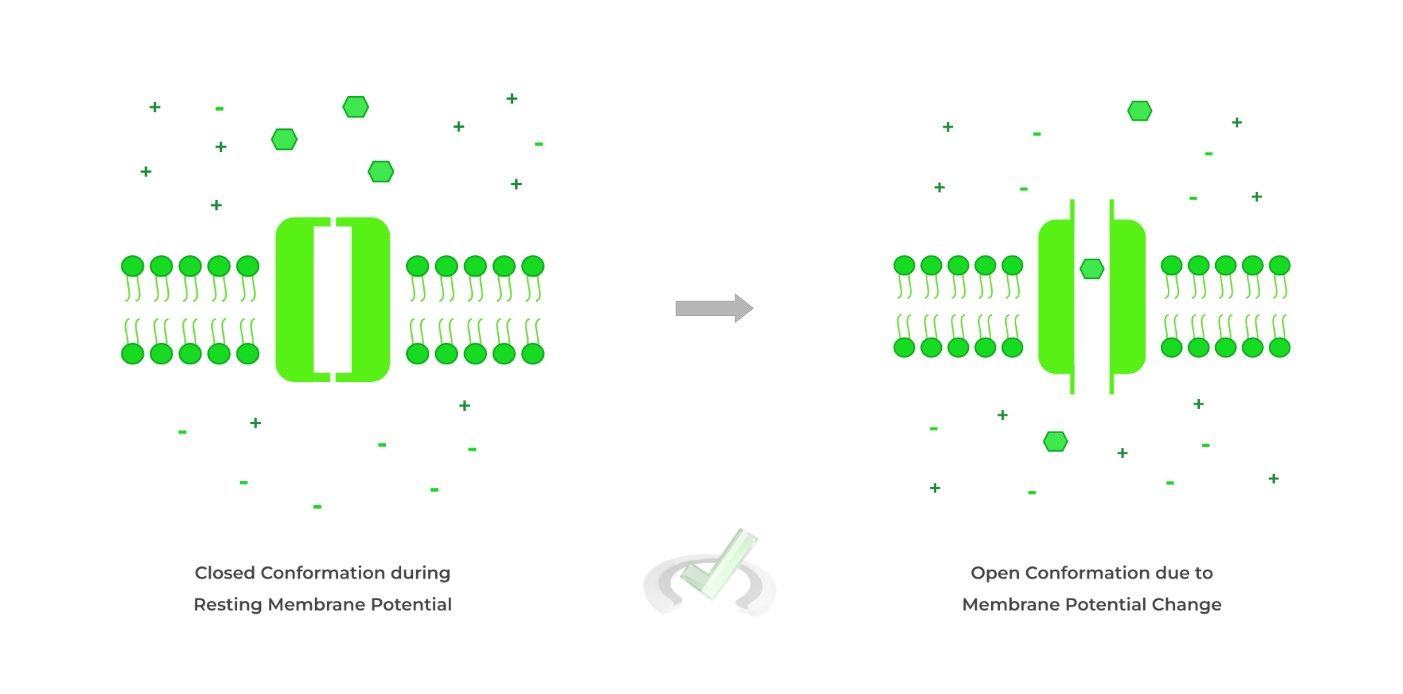
III. Voltage Gated
Finally, these ion channels open in response to a change in membrane potential from its resting membrane potential.
This often follows after ligand gated channel activation as the flow of ions from this channel can cause a change in membrane potential!
III. Bridge/Overlap
A great example of the ligand and voltage gated ion channels in physiological function is within a neuromuscular junction! Let’s quickly review some of the major components of the neuromuscular junction.
I. Ion Channels within the Neuromuscular Junction
The neuromuscular junction is a special type of synapse composed of a presynaptic motor neuron and a postsynaptic muscle cell. In order to initiate muscle contraction, an efferent signal is propagated through the motor neuron, causing the release of acetylcholine.
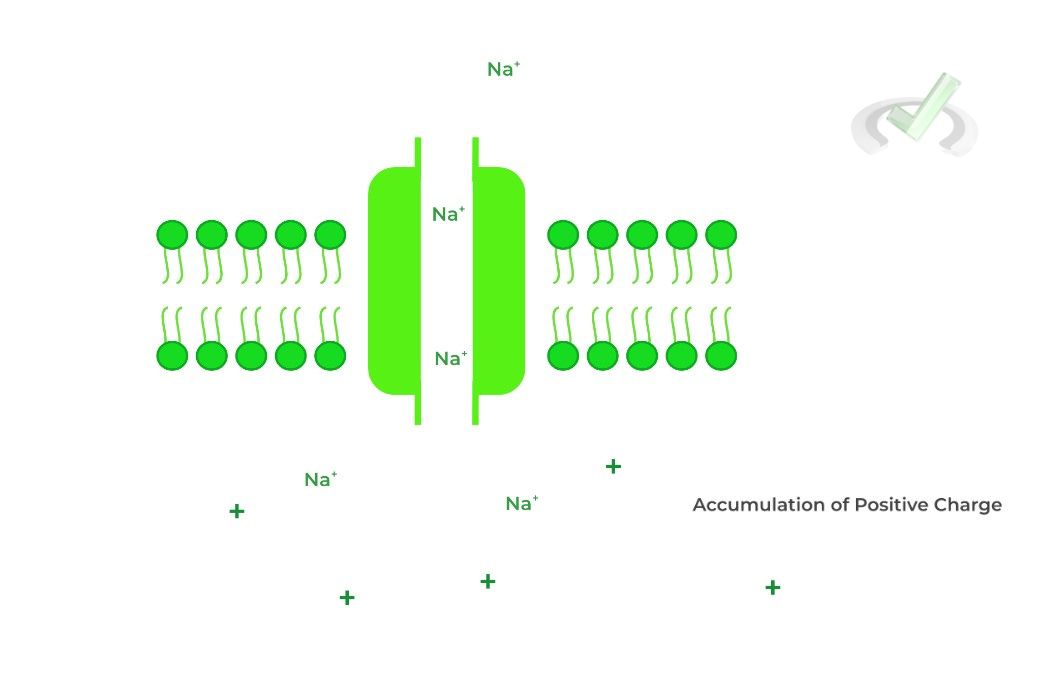
Acetylcholine then binds to the acetylcholine ligand gated ion channels on the muscle, which allows for flow of Na+ ions into the muscle cell.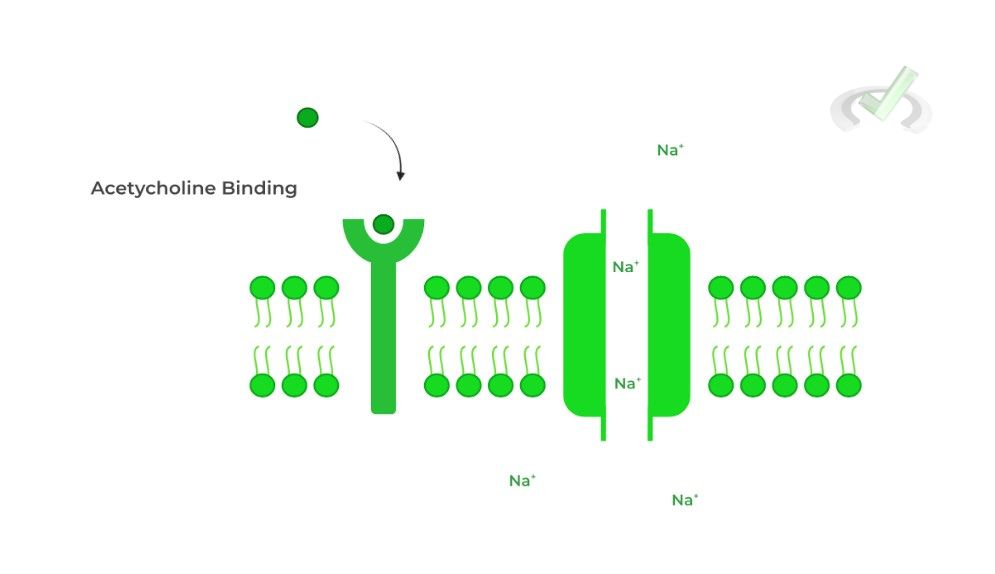
The influx of Na+ ions causes a change in membrane potential, which causes the Na+ voltage gated ion channels to open, which allows for the further depolarization and action potential
propagation needed for muscle contraction.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Receptors
Oftentimes, the binding of a signaling molecule to the receptor’s ligand binding domain allows for signal transduction, which can activate or inhibit a cellular pathway.
I. Enzyme Linked Receptors
The dual function of these proteins as receptors and enzymes comes from 2 domains: a ligand binding domain and a catalytic domain which has enzymatic activity!
II. G Protein Coupled Receptors
Picture these receptors as having 2 components: the actual heterotrimeric G protein, consisting of alpha, beta, and gamma subunits, and the receptor which connects to the G protein by a protein segment spanning the membrane 7 times.
Upon ligand binding, the inactive, GDP-alpha (𝛂) subunit is converted to an active GTP-alpha (𝛂) subunit, allowing for its dissociation and either activation or inhibition of adenylate cyclase.
If activated, adenylate cyclase catalyzes the conversion of AMP to cyclic AMP (cAMP), which goes on to activate other downstream products such as protein kinase A!B. Ion Channels
Through facilitating the flow of ions, a signal transduction can also occur depending on the circumstances!
I. Ungated
These are also known as leakage channels because they’re “always open”, causing ions to constantly leak and flow down their concentration gradient.
II. Ligand Gated
Similar to the G protein, think of these ion channels as having 2 components: the receptor and the ion channel. Upon ligand binding to the receptor, a conformational change occurs where the ion channel opens, allowing for the flow of ions!
III. Voltage Gated
This is somewhat similar to the ligand gated ion channels; however, instead of ligand binding causing a conformational change, a change in membrane potential is now the stimulus!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
After a ligand binds to a receptor’s ligand binding domain, the extracellular concentration of potassium ions decreases from 70mM to 20 mM. Which of the following best describes the possible type of biosignaling protein that was involved?
A. Enzyme Linked Receptor
B. Voltage Gated Ion Channel
C. Ungated Ion Channel
D. Ligand Gated Ion Channel
Ans. D
The above description is all concurrent with the function of a ligand gated ion channel. After the ligand binds to the receptor portion, the ion channel opens which allows for the flow of ions, explaining the decrease in extracellular potassium.
Sample Practice Question 2
Upon stimulation of a G protein coupled receptor, it’s found that there’s a buildup of AMP within the cell. What type of G protein, alpha subunit is being depicted: Gs or Gi?
Ans. Gi
Recall that the G protein, alpha subunit can either be stimulatory, activating adenylate cyclase, or inhibitory, inhibiting adenylate cyclase. The Gi class is being used because a buildup of AMP indicates that adenylate cyclase is being inhibited as its function is to convert AMP into cAMP when activated.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
