You’ve probably heard of what happened in Chernobyl and the devastation it caused. Even though some parts of the area looked generally okay, anything within the 18-mile radius of the power plant was closed off. The threat couldn’t be seen, yet people were advised to stay away. The phenomenon that caused a huge threat is because of one particular thing: Nuclear decay.
It is precisely as it sounds. Nuclear decay or radioactive decay is the death of the nucleus. During atomic decay, the nucleus undergoes tremendous change to make up for the instability within the nucleus.
Nuclear decay is interesting because we see how damaging it might be to society. Still, ironically, we use nuclear decay to treat illnesses that ordinary drugs cannot do. In the MCAT, you will be tested on how much you know about the fundamentals of nuclear decay, as it is an essential concept in understanding how we treat and diagnose diseases using specialized equipment.
I. Mechanism
We’ve previously discussed subatomic particles and how each element exists with a set number of protons. Each element of the same kind has the same number of protons but can also exist with different amounts of neutrons. Elements that exist with a different number of neutrons are called isotopes.
Isotopes can occur naturally or be synthesized. They can also be stable or unstable. Stable isotopes don’t decay fast, whereas unstable isotopes do otherwise. Isotopes generally differ by the number of neutrons present in their nucleus. When the neutron is significantly larger than the number of protons compared to the more stable variant of the element, the isotope becomes unstable. It will always want to make up for the imbalance between the natural forces in the atom, resulting in its subsequent decay.
A decay occurs when an unstable isotope has an imbalance of protons and neutrons. Instability results in the atom wanting to gain stability and it can do so by emitting an alpha particle or the transformation of a neutron.
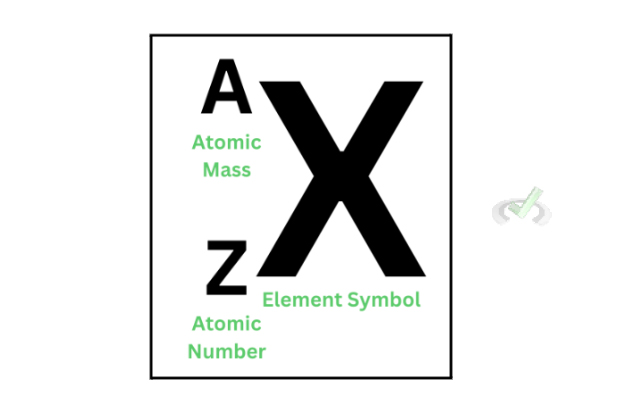
From this point on, we’ll use a naming convention to understand how this decay works. Refer to the image to the right to understand how we symbolize nuclear decay.
II. Alpha decay
Alpha decay is a type of nuclear decay that releases two protons and two neutrons, sometimes called a helium-4 (He-4) atom. In an unstable isotope, the nucleus has a significant imbalance due to the imbalance of neutrons. This imbalance leads to the nucleus wanting to lose some energy by releasing an alpha particle. When the nucleus releases an alpha particle, the atom loses 2 protons, which turns into a daughter nucleus with 2 fewer protons, making it into another different element.
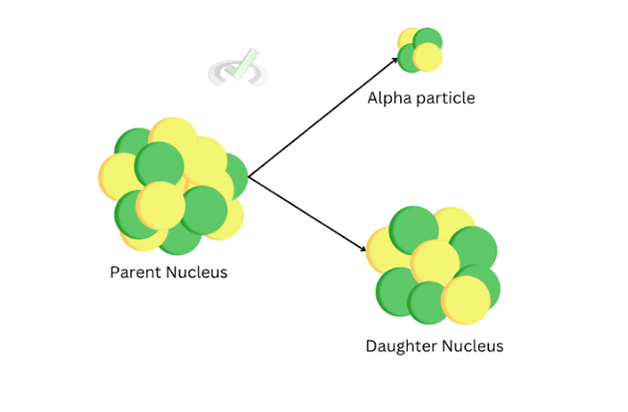
Taking the alpha decay of uranium-235, we see that the parent nucleus (U-235) transforms into the daughter nucleus (Th-231) and releases an alpha particle with 2 neutrons and 2 protons. When this happens, the daughter nucleus comes from an excited state to a stable state. This de-excitation state sometimes follows and releases a gamma ray as an energy release.

III. Beta Decay
A beta decay is a type of transformation that occurs in the neutron due to the imbalance in the proton-to-neutron ratio. During beta decay, a neutron makes up for this imbalance. It changes into a proton, or sometimes the proton itself changes into a neutron.
A. Beta Minus Decay
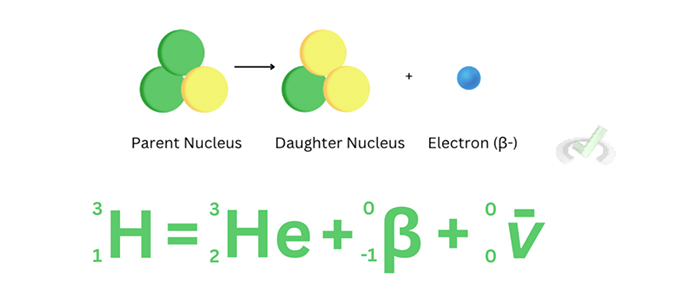
In a 𝜷- decay, the neutron turns into a proton. In this process, an electron and a particle with no charge (called an antineutrino) are released. During the decay of tritium (hydrogen-3), The parent nucleus turns into Helium 3 as a result of losing a beta-negative particle and the release of an antineutrino.
B. Beta Plus Decay
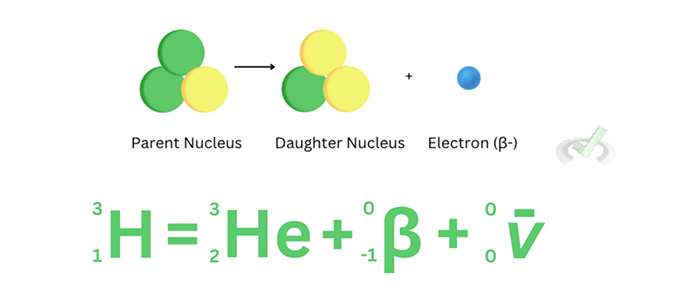
A 𝜷+ decay, the proton in the nucleus decays and turns into a neutron, which in turn releases a positive beta particle (also known as a positron) and a neutrino. In the beta plus decay of carbon-6, the parent nucleus turns into a Boron-10 daughter nucleus along with the release of a positive beta particle and a neutrino.
All of these subsequent decay may lead to the release of gamma rays. Similar to how the excitation from the daughter particle can result in the release of gamma rays, beta decay can similarly trigger gamma emission.
IV. Gamma Decay
Unlike alpha and beta decay, gamma decay does not change the number of protons and neutrons present. As mentioned before, during alpha and beta decay, the change from the parent nucleus to the daughter nucleus sometimes leaves the daughter nucleus in intense energy. Think of it this way: after losing a proton or changing into a ‘charged’ state, the nucleus will have a reserved energy after all those changes occur. This highly excited state will make the nucleus unstable due to the pent-up energy within the daughter nucleus. When this happens, energy gets released in the form of a gamma ray.
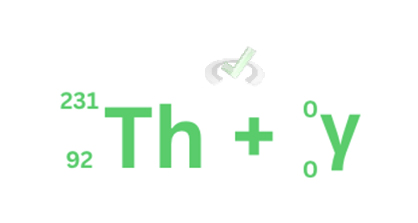
In the example below, nothing changes with the atomic mass and number of the daughter element. What happens during gamma decay is the release of energy in the form of gamma rays. Unlike alpha and beta rays, gamma rays cannot be stopped by thick walls. This is what makes the radiation coming from Chernobyl so dangerous. Gamma rays can penetrate essentially anything and as long as a lot of nuclear decay happens, gamma rays will always be released.
V. Conclusion
Nuclear decay or radioactive decay is the transformation of a nucleus into a different element as a result of instability within the nucleus. When the nucleus is unstable, it tends to do something to make up for that instability. One way of doing this is by alpha decay. During alpha decay, an alpha particle containing two protons and neutrons gets released from the parent nucleus, which in turn transforms the parent into a daughter nucleus with two fewer protons. On the other hand, beta decay involves the transformation of a proton or a neutron to rectify the instability.
There are two types of beta decay. One of which is beta minus decay which is when a neutron is converted into a proton along with the release of an electron and an antineutrino. Beta plus decay is a type of decay wherein the proton is converted into a neutron. A positron and a neutrino are released in the process. Gamma decay is the result of an energy release from a daughter nucleus. Too much exposure to gamma rays is highly dangerous as they can penetrate almost any surface. During nuclear decay, the nucleus of an atom changes and turns into a different element. Sometimes, the change from a parent to a daughter nucleus causes the daughter nucleus to have a high energy which it then releases to the environment as gamma rays.
VI. Key Terms
- Nuclear/radioactive decay - the transformation of an unstable nucleus as a result of energy release.
- Alpha decay - radioactive decay that results in the release of two protons and two neutrons.
- Beta decay - radioactive decay that involves the transformation of a proton into a neutron and a neutron into a proton.
- Gamma decay - the release of gamma rays as a result of an energy release from the daughter nucleus.
- Neutrino - a particle with minimal mass and neutral charge. A byproduct of beta minus decay.
- Antineutrino - the counterpart of a neutrino and also has a small mass and neutral charge. A byproduct of beta plus decay
- Positron - the positive counterpart of an electron containing the same mass as an electron but a positive charge. A byproduct of beta plus decay.
- Parent nucleus - the unstable nucleus that is the starting point of nuclear decay.
- Daughter nucleus - the parent nucleus after nuclear decay.
VII. Practice Questions
Sample Practice Question 1
Xenon-133 is used in specialized body scans to track pulmonary function. This gas is inhaled and undergoes beta decay which helps in imaging. Determine the daughter isotope that will be produced in the beta decay of Xe-133.
A. Cs-132
B. Xe-129
C. Cs-133
D. Xe-132
Ans. C
Sample Practice Question 2
Another isotope used in the medical field is Radon-122. This element is used to treat cancer due to the byproduct it emits during decay. If Rn-222 decays into Po-218, what type of decay does the isotope go through?
A. Alpha decay
B. Beta plus decay
C. Beta minus decay
D. Gamma decay
Ans. A







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot