Organic chemistry involves the study of carbon-containing compounds. With millions of possible organic molecules, a systematic naming method is essential.
The IUPAC nomenclature provides rules for naming organic compounds based on their structure. This allows chemists to deduce the structure from the name.
Before the IUPAC system, organic molecules had common names that were often confusing. In 1892, the first International Congress of Chemists created a systematic method to name organic compounds. This method evolved into the current IUPAC system.
I. Basics of IUPAC Nomenclature
The IUPAC naming system involves several steps and rules for accurately naming organic compounds. These steps ensure that each molecule's name reflects its structure and functional groups.

Identifying the Longest Carbon Chain
First, identify the longest continuous chain of carbon atoms. This chain determines the base name of the compound. For example:
- Meth- for one carbon
- Eth- for two carbons
- Prop- for three carbons
- But- for four carbons
Numbering the Carbon Chain
Next, number the carbon atoms in the longest chain. Start from the end nearest to the first substituent. This ensures the substituents have the lowest possible numbers.
Identifying and Naming Substituents
Substituents are groups of atoms attached to the main carbon chain. Common substituents include:
- Methyl (CH₃)
- Ethyl (C₂H₅)
- Propyl (C₃H₇)
Assembling the Name
Combine the names of the substituents with the base name of the carbon chain. List the substituents in alphabetical order, each preceded by its position number.
Examples:
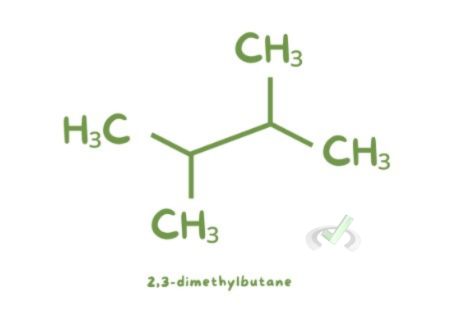
- 2,3-dimethylbutane: This name indicates a four-carbon chain (butane) with two methyl groups on the second and third carbons.
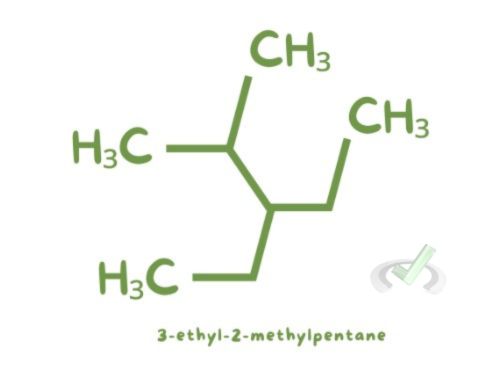
- 3-ethyl-2-methylpentane: This name indicates a five-carbon chain (pentane) with an ethyl group on the third carbon and a methyl group on the second carbon.
II. Functional Groups and Their Influence on Naming
Functional groups are specific groups of atoms within molecules that have characteristic properties. They play a significant role in the reactivity and naming of organic compounds.
Common Functional Groups
- Hydroxyl Group (-OH): Alcohols
- Carbonyl Group (C=O): Aldehydes and Ketones
- Carboxyl Group (-COOH): Carboxylic Acids
- Amino Group (-NH₂): Amines
Naming Compounds with Functional Groups
When naming compounds with functional groups, identify the main functional group and use its suffix in the compound's name.
Examples:
- Alcohols: Replace the -e at the end of the alkane name with -ol (e.g., methane becomes methanol).
- Aldehydes: Replace the -e with -al (e.g., ethane becomes ethanal).
- Ketones: Replace the -e with -one (e.g., propane becomes propanone).
Naming Compounds with Multiple Functional Groups
When a molecule contains more than one functional group, the functional group with the highest priority determines the suffix, and other groups are named substituents.
Examples:
- 4-hydroxybutanoic acid: This name indicates a four-carbon chain with a carboxyl group (highest priority) and a hydroxyl group on the fourth carbon.
- 2-amino-3-hydroxybutanoic acid: The carboxylic acid is the highest priority group, so it determines the suffix. The amino and hydroxyl groups are named as substituents.
Priority Order
The priority order of common functional groups from highest to lowest is:
- Carboxylic Acids
- Anhydrides
- Esters
- Aldehydes
- Ketones
- Alcohols
- Amines
- Alkenes
- Alkynes
- Alkanes
III. Special Cases in Nomenclature
Cyclic Compounds
For cyclic compounds, use the prefix cyclo- before the base name of the carbon chain. Number the ring to give substituents the lowest possible numbers.
Examples:
- Cyclohexane: A six-carbon ring.
- 1,2-dimethylcyclopentane: A five-carbon ring with two methyl groups on the first and second carbons.
Aromatic Compounds
Aromatic compounds, such as benzene derivatives, follow specific rules. When naming benzene derivatives, use prefixes like ortho- (1,2-), meta- (1,3-), and para- (1,4-) to indicate the substituents’ position on the benzene ring.
Examples:
- 1,3-dimethylbenzene: Also called m-xylene, indicates two methyl groups on the first and third positions of a benzene ring.
- 2-nitrotoluene: Indicates a benzene ring with a methyl group (toluene) and a nitro group in the second position.
IV. Scientific Significance of Organic Nomenclature
Let's explore in detail how understanding organic nomenclature connects to broader chemistry ideas. This enriches our knowledge of chemical behavior and its applications in various fields.
Structure-Function Relationship
The name of an organic compound provides insight into its structure. This is crucial for understanding its chemical behavior. Knowing the structure helps predict the physical and chemical properties of the compound.
Synthesis and Reactions
Accurate naming is essential in organic synthesis and reactions. Chemists need precise names to understand the steps involved in creating or transforming organic molecules. Understanding the structure and name of a reactant is vital for predicting the products of a chemical reaction.
Biological Significance
Many biologically important molecules are organic compounds. Proper naming helps in the study of biochemistry and pharmacology. Knowing the structure and name of amino acids and nucleotides is crucial for understanding protein synthesis and genetic information.
Relevance in Drug Design
Organic nomenclature is critical in drug design and development. Accurate naming aids in the identification and synthesis of pharmaceutical compounds. Understanding the structure and functional groups of a drug molecule is vital for predicting its biological activity and interactions.
For example, the drug acetaminophen (Tylenol) is named systematically as N-(4-hydroxyphenyl)acetamide. This name provides information about its structure and functional groups. It is important for its synthesis and understanding its pharmacological properties.
V. Conclusion and Key Terms
Understanding the naming fundamentals of organic chemistry molecules involves grasping several key concepts and principles. Let's review:
Key Terms
- IUPAC Nomenclature: Standardized system for naming organic compounds.
- Substituent: An atom or group of atoms attached to the main carbon chain.
- Functional Group: A specific group of atoms within molecules that have characteristic properties.
- Base Name: The name derived from the longest continuous carbon chain in the molecule.
- Priority Order: The order of precedence of functional groups when naming compounds with multiple functional groups.
VI. Practice Questions
Sample Practice Question 1
What is the base name of a compound with a four-carbon chain?
A. Prop-
B. But-
C. Pent-
D. Hex-
Ans. B
The prefix "but-" indicates a four-carbon chain in the IUPAC naming system.
Sample Practice Question 2
How is a compound with a hydroxyl group on the second carbon of a propane chain named?
A. 1-propanol
B. 2-propanol
C. 1-propene
D. 2-propene
Ans. B
The hydroxyl group is on the second carbon of the propane chain, so the compound is named 2-propanol.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot