In organic chemistry, common names are often used alongside IUPAC names. Common names come from history or practical use.
IUPAC names follow specific rules. Substituents are groups attached to the main carbon chain. Their names change based on their structure and the type of compounds they form. Substituents can change the properties and reactions of organic molecules.
I. Common Names of Organic Compounds
Some organic compounds are more often called by their common names. Here are a few important ones to know.
A. Alkanes and Alkenes
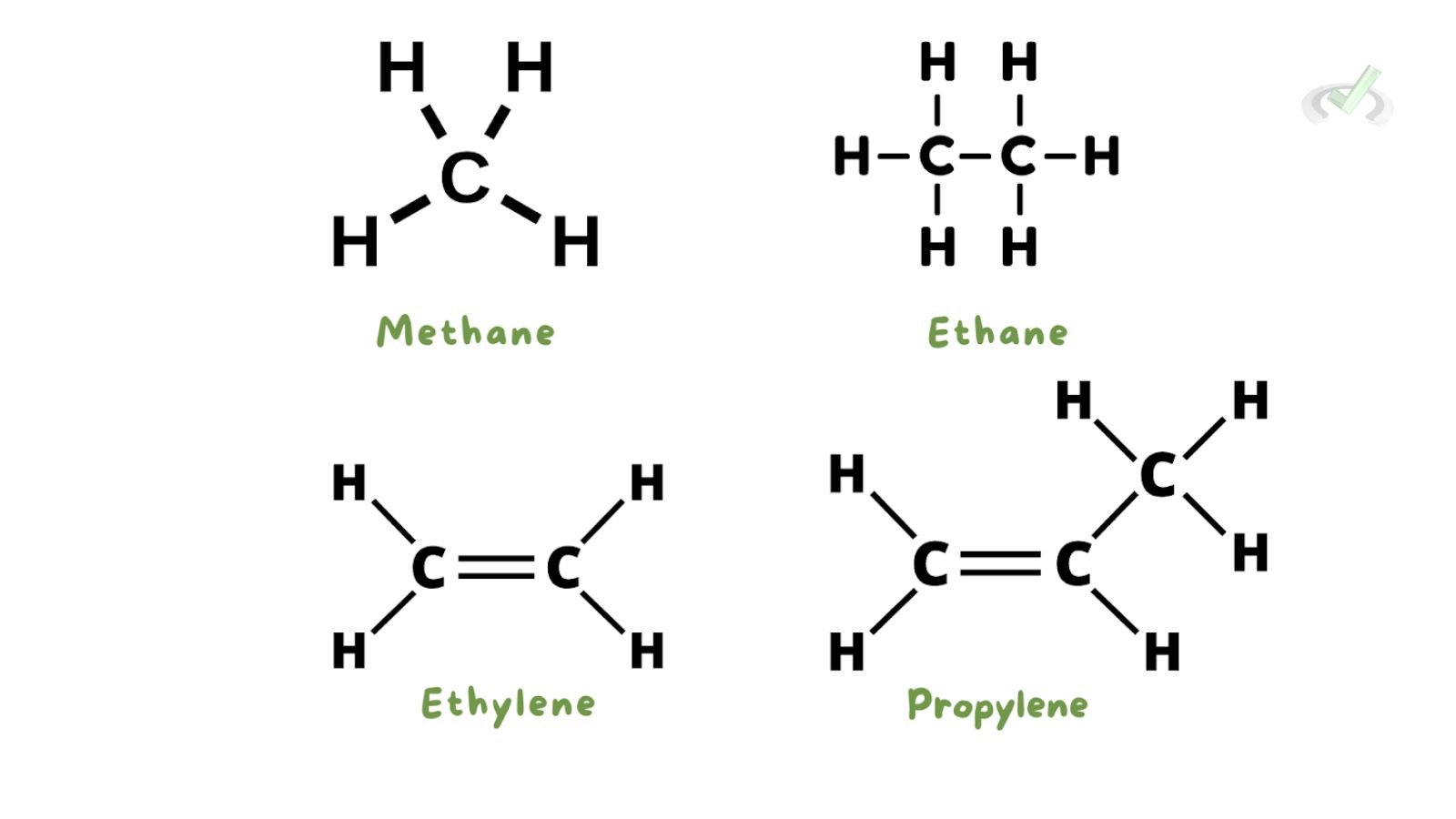
- Methane (CH4): The simplest alkane, known as natural gas.
- Ethane (C2H6): A two-carbon alkane used to make ethylene.
- Ethylene (C2H4): Also known as ethene, an important industrial chemical.
- Propylene (C3H6): Also known as propene, used in making plastics.
B. Alcohols
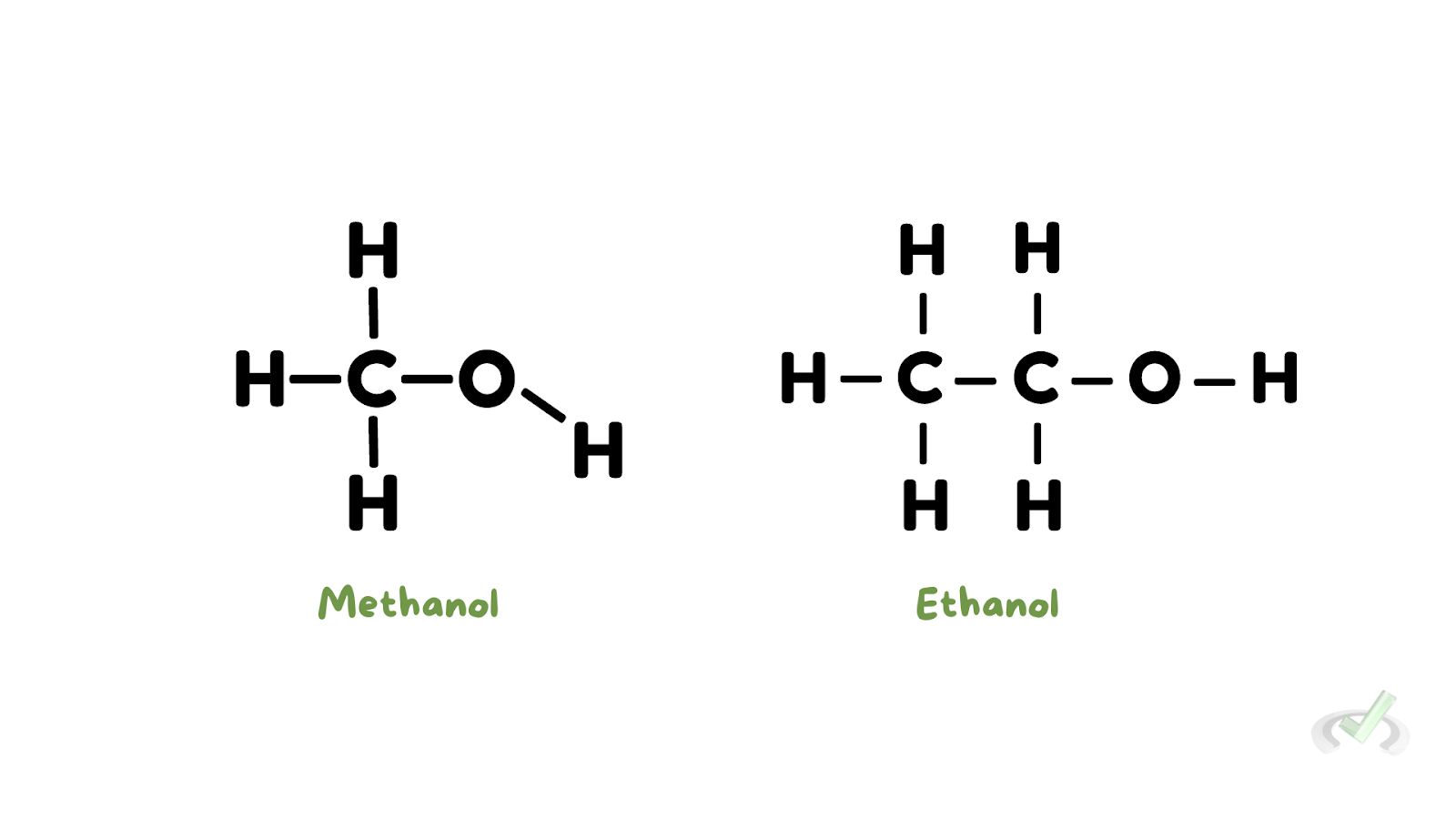
- Methanol (CH3OH): Also known as wood alcohol, used as a solvent and antifreeze.
- Ethanol (C2H5OH): Commonly called alcohol, it is found in alcoholic drinks.
- Isopropanol (C3H8O): Also known as isopropyl alcohol, used as a disinfectant.
C. Carboxylic Acids
- Formic Acid (HCOOH): The simplest carboxylic acid found in ant venom.
- Acetic Acid (CH3COOH): Known as vinegar when diluted.
- Benzoic Acid (C7H6O2): An aromatic carboxylic acid used as a preservative.
D. Ketones and Aldehydes
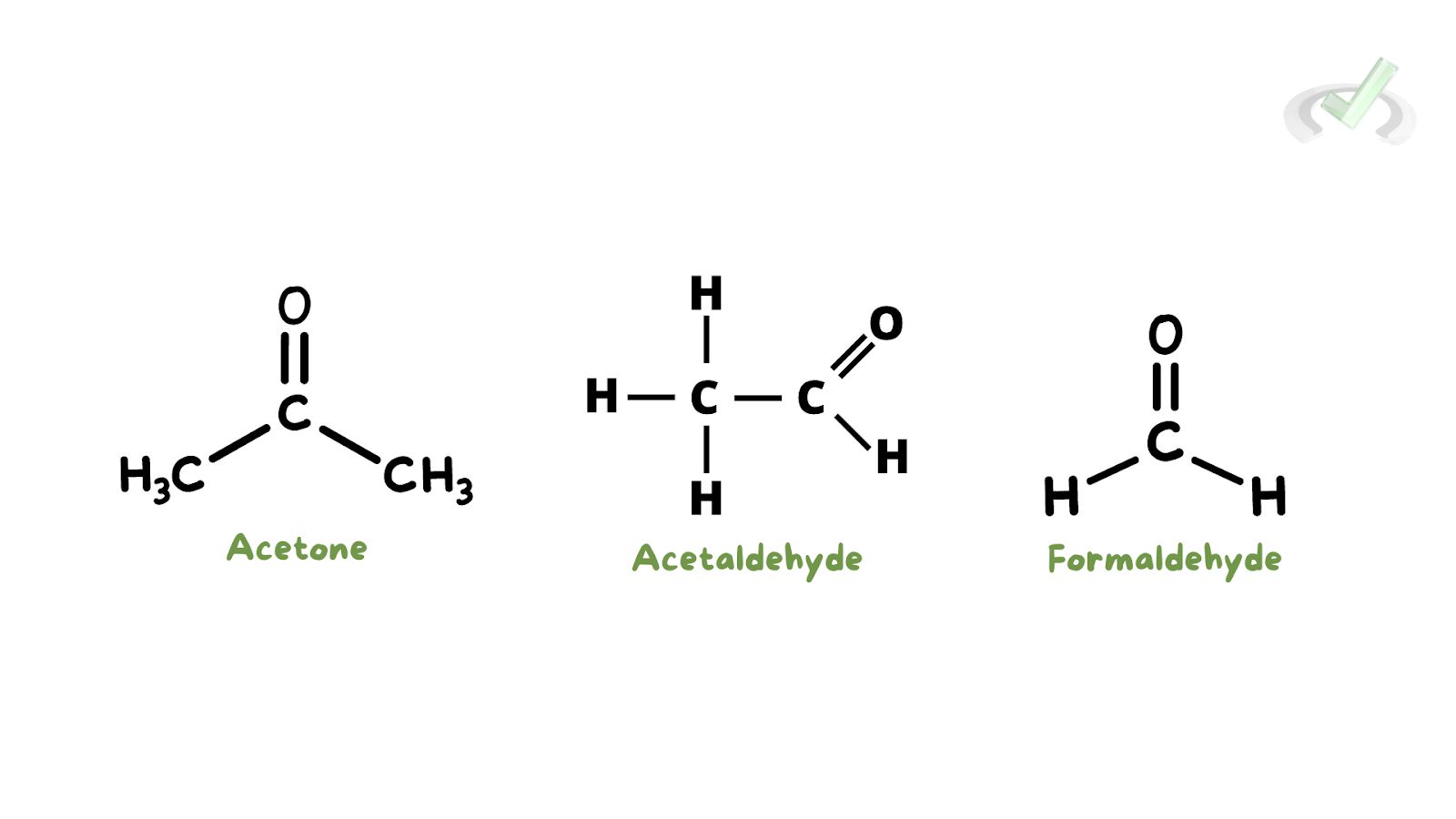
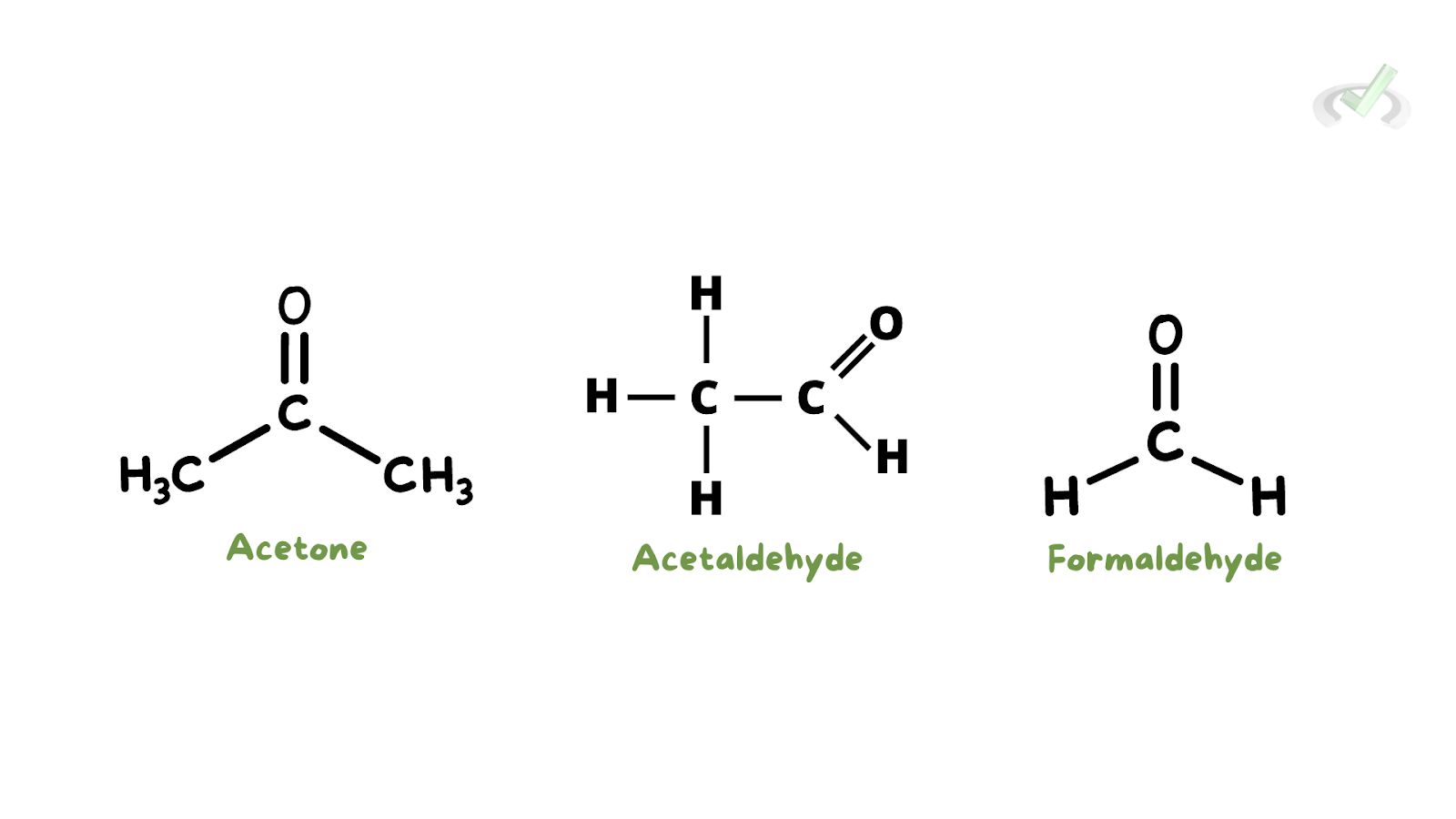
- Acetone (C3H6O): The simplest ketone, used as a solvent in nail polish remover.
- Formaldehyde (CH2O): The simplest aldehyde used in embalming and disinfection.
- Acetaldehyde (C2H4O): Used to make acetic acid.
II. Important Substituents and Their Names
Substituents are groups attached to the main carbon chain. They play a crucial role in determining the properties and reactions of organic molecules. Here are some important ones.
A. Alkyl Groups
- Methyl Group (-CH3): The simplest alkyl group found in many compounds.
- Ethyl Group (-C2H5): A two-carbon alkyl group common in organic molecules.
- Propyl Group (-C3H7): Can be straight-chain (n-propyl) or branched (isopropyl).
B. Halogen Substituents
- Fluoro Group (-F): Adding fluorine to hydrocarbons increases stability and reactivity.
- Chloro Group (-Cl): Found in many pesticides and solvents.
- Bromo Group (-Br): Can be straight-chain (n-propyl) or branched (isopropyl).
- Iodo Group (-I): Less common but used in organic synthesis and medical imaging.
C. Hydroxyl and Amino Groups
- Hydroxyl Group (-OH): Found in alcohols and phenols, which are important for hydrogen bonding.
- Amino Group (-NH2): Found in amines and amino acids, basic in nature and important in proteins.
D. Carbonyl Groups
- Aldehyde Group (-CHO): Found at the end of carbon chains in aldehydes, highly reactive.
- Ketone Group (>C=O): Found within carbon chains in ketones, it is also reactive and important in metabolism.
E. Aromatic Substituents
- Phenyl Group (-C6H5): A benzene ring attached to a larger molecule, common in drugs and plastics.
- Benzyl Group (-CH2C6H5): A benzene ring attached via a -CH2- group found in fragrances and solvents.
- Nitro Group (-NO2): Strongly electron-withdrawing, used in explosives and reactions.
- Methoxy Group (-OCH3): An ether group attached to an aromatic ring affects reactivity and solubility.
III. Applications and Reactions Involving Common Names and Substituents
Understanding common names and substituents helps predict the behavior of organic molecules in reactions. Here are some examples.
A. Reactions with Alkyl Halides
Alkyl halides (R-X) are used in nucleophilic substitution and elimination reactions. The type of alkyl group and halogen affects the reaction. For example, tertiary alkyl halides often undergo elimination reactions. Primary alkyl halides usually favor substitution reactions.
B. Alcohols and Their Reactions
Alcohols can dehydrate to form alkenes. They can also oxidize to form aldehydes, ketones, or carboxylic acids. Knowing the structure of alcohols, like methanol or ethanol, helps predict their behavior in reactions. For example, primary alcohols oxidize to form aldehydes. Secondary alcohols oxidize to form ketones.
C. Aromatic Compounds
Aromatic compounds like benzene undergo substitution reactions. The role of substituents like phenyl or benzyl groups is crucial in predicting the products. Electron-donating groups (e.g., -OH, -CH3) activate the benzene ring for substitution. Electron-withdrawing groups (e.g., -NO2, -COOH) deactivate it.
D. Carbonyl Compounds
Aldehydes and ketones are very reactive in nucleophilic addition reactions. Recognizing carbonyl compounds like acetone and formaldehyde helps predict their behavior. Aldehydes are more reactive than ketones due to less steric hindrance and greater electron deficiency at the carbonyl carbon.
IV. Connecting to Broader Organic Chemistry Concepts
Knowing common names and substituents is just the beginning. These concepts connect to broader topics in organic chemistry, such as reaction mechanisms, synthesis, and functional group transformations.
A. Synthesis and Functional Group Transformations
Understanding substituents helps in designing synthetic pathways for complex molecules. Knowing the reactivity of groups like hydroxyl and carbonyl helps plan multi-step syntheses. For example, converting an alcohol to a ketone might involve oxidation followed by nucleophilic addition.
B. Reaction Mechanisms
The behavior of molecules in reactions is influenced by their substituents. Electron-donating or electron-withdrawing groups affect the stability of reaction intermediates and transition states. This knowledge is crucial for understanding why certain reactions proceed faster or with higher yields.
C. Biochemical Relevance
Many important biological molecules have specific common names and substituents. For example, amino acids have amino and carboxyl groups. These substituents influence their behavior in biochemical reactions. Understanding these groups helps in studying enzyme mechanisms and metabolic pathways.
V. Wrap-Up and Key Terms
Understanding the common names and substituents in organic chemistry involves grasping several key concepts and principles. Let's review:
- Common Names: Historical or practical names of organic compounds.
- Substituents: Groups attached to the main carbon chain.
- Alkyl Groups: Methyl, ethyl, propyl groups.
- Halogen Substituents: Fluoro, chloro, bromo, iodo groups.
- Hydroxyl Group (-OH): Found in alcohols and phenols.
- Amino Group (-NH2): Found in amines and amino acids.
- Carbonyl Groups: Aldehyde and ketone groups.
- Aromatic Substituents: Phenyl, benzyl, nitro, and methoxy groups.
VI. Practice Questions
Sample Practice Question 1
What is the common name for CH3OH?
A) Ethanol
B) Methanol
C) Propanol
D) Isopropanol
Ans. B
Methanol is commonly known as wood alcohol and is used as a solvent and antifreeze.
Sample Practice Question 2
Which substituent is represented by the group -NH2?
A) Hydroxyl
B) Methyl
C) Amino
D) Carbonyl
Ans. C
The -NH2 group is called an amino group and is found in amines and amino acids.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot