Mass spectrometry (MS) is a method utilized in the field of organic chemistry for analyzing the mass and structure of molecules. It involves ionizing chemical compounds to produce charged molecules or molecule fragments and then assessing their mass-to-charge ratios.
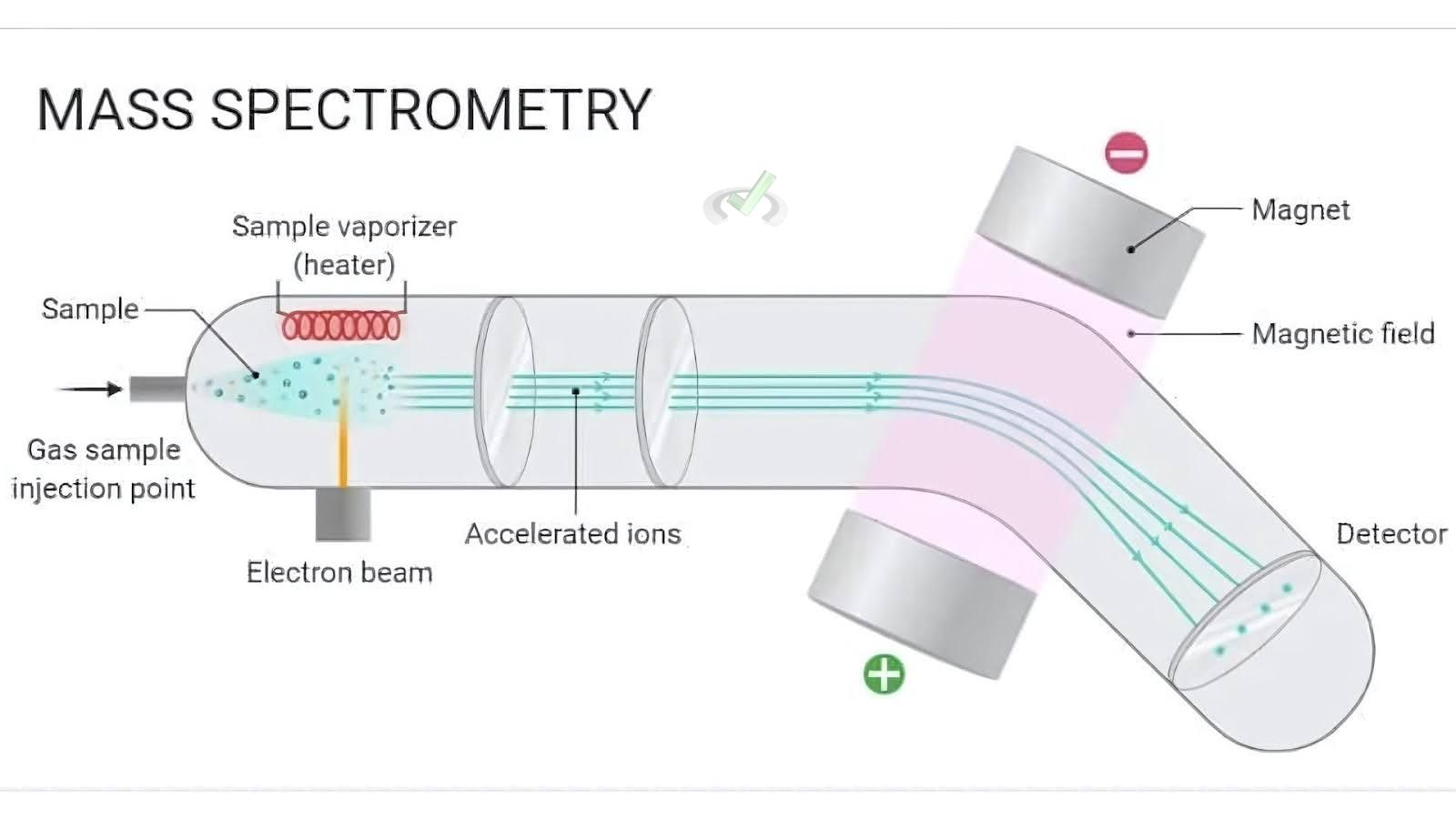
I. Basics of Mass Spectrometry
A. Ionization
Ionization converts molecules into ions by adding or removing charged particles like electrons. In mass spectrometry, ionization is crucial because electric and magnetic fields allow the molecules to be manipulated.
Example: When a methane molecule (CH₄) is ionized, it can lose an electron to form a CH₄⁺ ion.
B. Mass-to-Charge Ratio (m/z)
The mass-to-charge ratio, or m/z, is a critical concept in mass spectrometry. It is the ratio of an ion's mass to its charge. By measuring m/z, we can determine the mass of the ions produced during ionization.
II. Types of Ionization Techniques
A. Electron Ionization (EI)
In electron ionization, high-energy electrons collide with a molecule, causing it to lose an electron and form a positive ion.
Example: When benzene (C₆H₆) undergoes electron ionization, it forms a C₆H₆⁺ ion.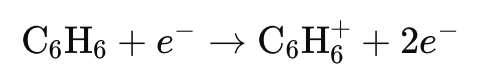
B. Chemical Ionization (CI)
In chemical ionization, a reagent gas (like methane) is ionized first, and then this ionized gas ionizes the sample molecules. This technique is softer than electron ionization, leading to less fragmentation.
Example: Methane (CH₄) ionizes to form CH₅⁺, which then ionizes a sample molecule (M) to form (M+H)⁺.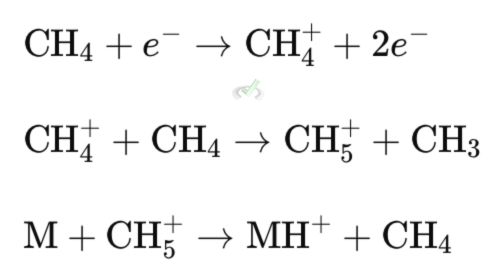
III. Fragmentation Patterns
A. Molecular Ion Peak (M⁺)
In chemical ionization, a reagent gas (like methane) is ionized first, and then this ionized gas ionizes the sample molecules. This technique is softer than electron ionization, leading to less fragmentation.
Example: For ethanol (C₂H₅OH), the molecular ion peak appears at m/z 46, corresponding to C₂H₅OH⁺.B. Base Peak
The base peak is considered the tallest peak in the mass spectrum, representing the most abundant ion. It is often a fragment of the original molecule.
Example: In the mass spectrum of ethanol, the base peak might be at m/z 31, corresponding to the fragment CH₂OH⁺.IV. Interpreting Mass Spectra
A. Identifying the Molecular Ion
To identify the molecular ion, look for the peak with the highest m/z value that corresponds to the compound's molecular weight.
Example: For benzene (C₆H₆), the molecular ion peak is at m/z 78.B. Analyzing Fragmentation Patterns
Fragmentation patterns help determine the structure of the molecule. Each fragment gives clues about the bonds present in the original molecule.
Example: In the mass spectrum of butane (C₄H₁₀), common fragments include C₄H₉⁺ (m/z 57) and C₂H₅⁺ (m/z 29).V. Applications of Mass Spectrometry
A. Structural Elucidation
Mass spectrometry helps identify the structure of unknown compounds by analyzing their mass and fragmentation patterns. One example is determining the structure of a new drug by identifying its molecular ion and fragmentation pattern.
B. Quantitative Analysis
Mass spectrometry can quantify the amount of a compound in a sample by measuring the intensity of specific ions. For instance, it can measure the concentration of caffeine in a blood sample.
VI. Connecting Mass Spectrometry to Broader Organic Chemistry and Biochemistry Concepts
A. Molecular Weight Determination
Understanding the molecular ion peak helps determine a compound's molecular weight, which is essential in determining its molecular formula.
Example: Using the molecular ion peak at m/z 180 to identify the molecular weight of glucose (C₆H₁₂O₆).B. Fragmentation Mechanisms
Knowing common fragmentation mechanisms aids in predicting the structure of unknown fragments.
Example: Predicting the formation of a CH₃⁺ fragment (m/z 15) from the breakdown of ethane (C₂H₆).C. Biochemical Relevance
Mass spectrometry is crucial in proteomics, the study of proteins. It helps identify and characterize proteins based on their mass and peptide fragments, which is essential in understanding enzyme functions, signaling pathways, and disease mechanisms.
Example: Using mass spectrometry to identify a protein by its peptide mass fingerprint.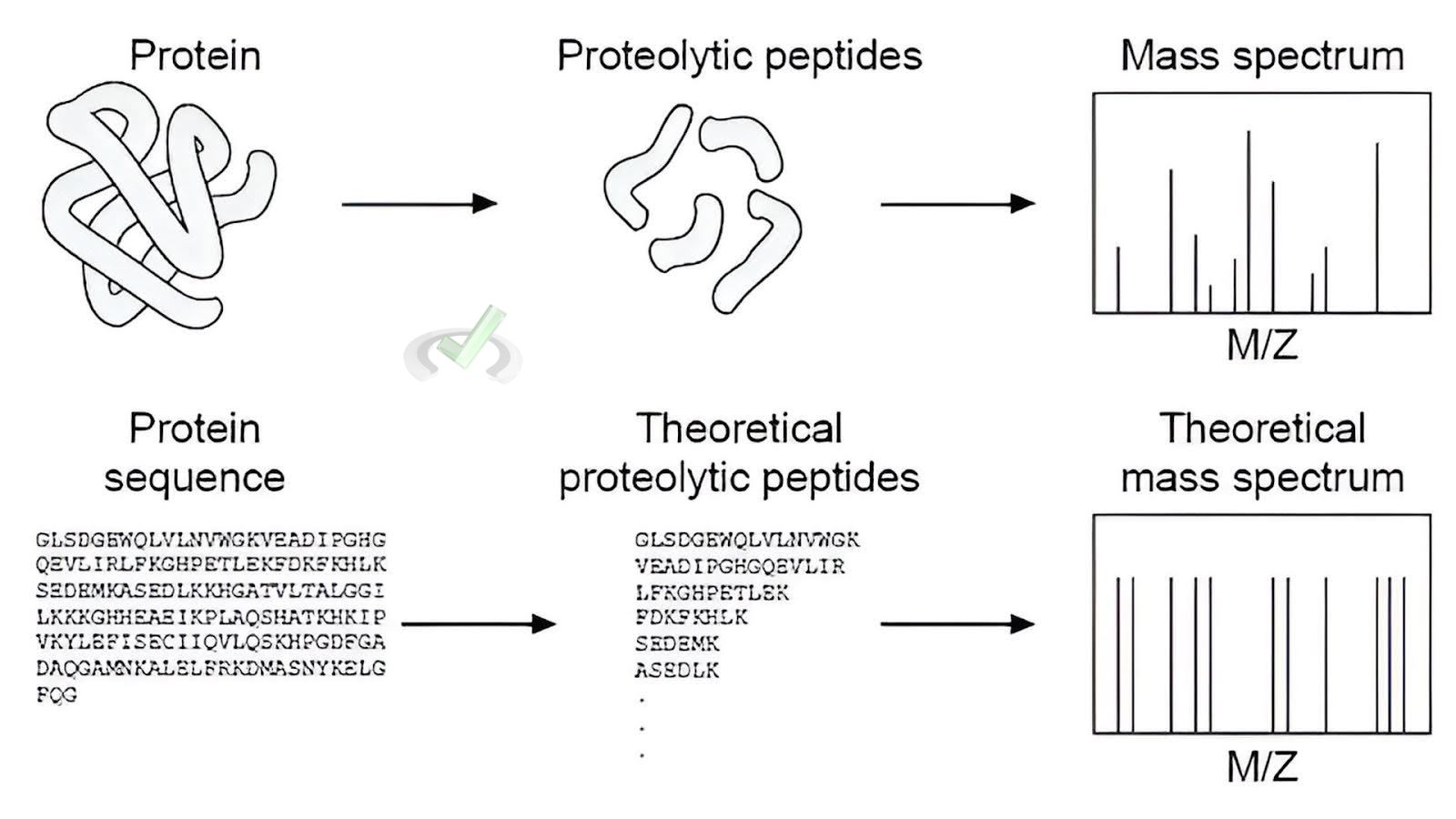
D. Metabolic Pathways
Mass spectrometry is also used to study metabolic pathways. It can track metabolite changes, which helps understand how cells respond to various conditions.
Example: Analyzing metabolites in the citric acid cycle to study cell energy production.E. DNA Sequencing
DNA sequencing uses advanced mass spectrometry techniques. These techniques help determine the sequence of nucleotides in DNA, which is crucial for genetic research and medical diagnostics.
Example: Using mass spectrometry to sequence DNA and identify genetic mutations related to diseases.VII. Wrap-Up and Key Terms
Understanding mass spectrometry involves several key concepts:
Key Terms:
Ionization: The process of forming ions by adding or removing charged particles.
Mass-to-Charge Ratio (m/z): An ion's mass ratio to its charge.
Molecular Ion Peak (M⁺): The peak representing the intact ionized molecule.
Base Peak: The tallest peak representing the most abundant ion.
VIII. Practice Questions
Sample Practice Question 1
What does the molecular ion peak represent in a mass spectrum?
A) The most abundant ion
B) The ion with the highest m/z value
C) The ion with the lowest m/z value
D) The ion formed by losing two electrons
Ans. B
The molecular ion peak represents the intact ionized molecule with the highest m/z value.
Sample Practice Question 2
What is the purpose of ionization in mass spectrometry?
A) To fragment the molecule
B) To form charged particles for manipulation
C) To measure the molecule's color
D) To change the molecule's state
Ans. B
Ionization converts molecules into ions, allowing them to be manipulated by electric and magnetic fields for measurement.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot